Introduction of Mercury
Mercury, known by its chemical symbol Hg and atomic number 80, is one of the most unique elements on the periodic table. Unlike most metals, mercury exists in a liquid state at room temperature. It has fascinated scientists for centuries due to its unusual physical properties, widespread use in ancient and modern times, and serious toxicological concerns. Mercury’s name originates from the Roman deity Mercury, a swift messenger, which might reflect the liquid metal’s fast-moving nature. This topic explores the sources of mercury, its properties, applications, the compounds it forms, the extraction and purification process, and some interesting facts.
Table of Contents
Toggle
1. Ores of Mercury
Mercury is a relatively rare element in the Earth’s crust, typically found in mineral ores. The most significant and primary ore of mercury is cinnabar (HgS), a brilliant red or brownish-red mineral that has been mined for centuries. Other, much rarer mercury-containing ores include livingstonite (HgSb₄S₈) and calomel (Hg₂Cl₂).
Cinnabar (HgS):
- Composition: Mercury sulfide (HgS).
- Appearance: Typically bright red, brownish-red, or black. In natural form, cinnabar is often used as a pigment.
- Location: Cinnabar deposits are found worldwide, with significant reserves in Spain, Italy, Russia, and parts of the United States.
2. Properties of Mercury
Mercury’s unusual properties make it stand out among the elements. Below are some of the key physical and chemical properties:
Physical Properties:
- State: Liquid at room temperature, a rare feature for metals.
- Density: Very dense, with a density of 13.534 g/cm³. It is approximately 13 times heavier than water.
- Melting Point: -38.83°C. Mercury is one of the few elements that is liquid even at sub-zero temperatures.
- Boiling Point: 356.73°C, which makes mercury easily vaporizable when heated.
- Surface Tension: High surface tension allows mercury to form spherical droplets.
- Electrical Conductivity: Like most metals, mercury is a good conductor of electricity.
Chemical Properties:
- Action with air: it forms a mercuric oxide when reacted with air at a temperature of 623K.
Hg+O2⟶HgO
- Action with aquaregia: mercury gets dissolved when reacted with aquaregia.
HNO3+3HCl+Hg⟶NOCl+HgCl2+2H2O
- Action with sulphur: it reacts with sulphur to form a mercuric sulphide.
- Reaction with metal: it reacts with metals to form the amalgam.
- Reaction with halogen: It reacts with halogens to form halides.
Hg+Cl2⟶HgCl2(limited amount of mercury)
- Tailing of mercury: when mercury is exposed to ozone, it superficially gets oxidized and loses its meniscus, it is called tailing of mercury. During this process dark grey-colored tail of mercurous oxide is formed.
2Hg+O3⟶Hg2O+O2
3. Applications and Uses of Mercury
Despite its toxic nature, mercury has historically been used in a wide range of applications due to its unique properties.
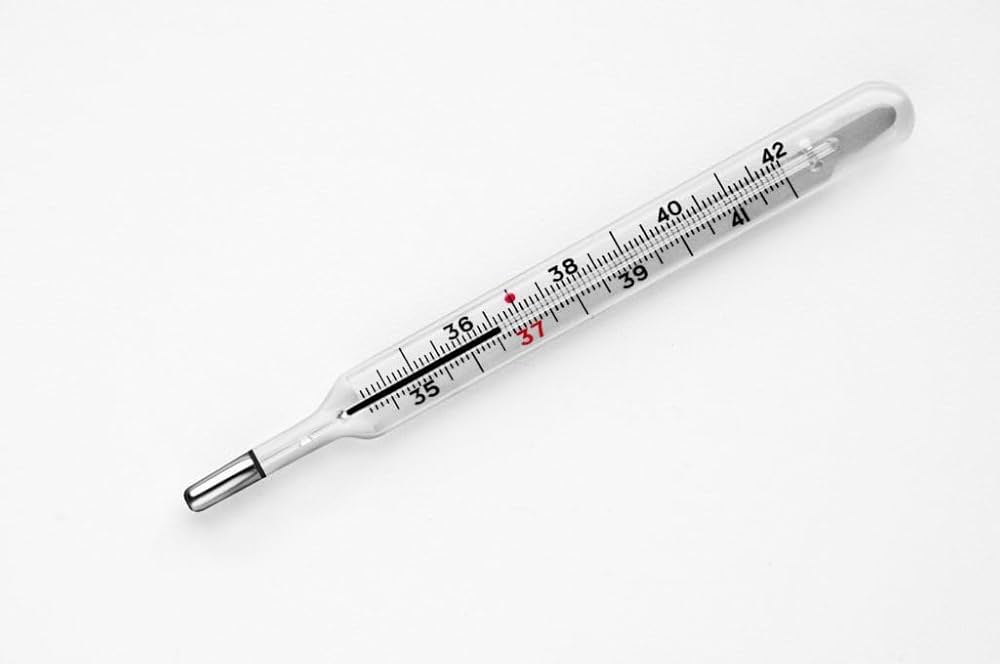
a. Thermometers and Barometers
Mercury expands uniformly with temperature, making it ideal for use in thermometers. Its high density and non-reactivity also make it useful in barometers, devices that measure atmospheric pressure. Though digital devices have replaced mercury-based instruments, these applications were once widespread.
b. Electrical and Electronic Devices
Mercury is used in mercury-vapor lamps, fluorescent lamps, and electrical switches. It conducts electricity well and is used in some older electrical switches and relays, particularly those exposed to extreme conditions.
c. Dental Amalgams
Mercury’s ability to form strong amalgams with silver, tin, and copper made it an essential component of dental fillings in the past. However, concerns over mercury’s toxicity have led to a significant reduction in its use for this purpose.
d. Chlor-Alkali Industry
Mercury is employed in the chlor-alkali process, where it is used to produce chlorine gas and caustic soda (sodium hydroxide) by electrolysis of brine.
e. Gold and Silver Mining
Mercury is used in a process called amalgamation to extract gold and silver from ores. The metal binds to the precious metals and forms an amalgam, which is then heated to vaporize the mercury, leaving behind pure gold or silver.
4. Compounds of Mercury
Mercury forms a variety of compounds, often classified by its oxidation states: mercurous (Hg⁺) and mercuric (Hg²⁺).
a. Mercurous Compounds (Hg(I)):
- Mercurous Chloride (Hg₂Cl₂): Also known as calomel, this white crystalline solid was once used as a laxative and in medicinal applications. However, due to its toxicity, its use has been largely discontinued.
b. Mercuric Compounds (Hg(II)):
- Mercuric Oxide (HgO): A bright red or yellow solid used in the production of mercury vapor and batteries. It decomposes to release oxygen when heated, which has made it useful in laboratory experiments.
- Mercuric Chloride (HgCl₂): A highly toxic and corrosive compound used historically as a disinfectant. Today, its use is limited to industrial applications due to its toxicity.
- Methylmercury (CH₃Hg): An organic compound of mercury that forms in aquatic environments through bacterial action. It is one of the most toxic forms of mercury and is a significant environmental concern because it bioaccumulates in fish and shellfish.
5. Extraction and Purification of Mercury
The most common method for extracting mercury from cinnabar ore involves roasting. The steps in the process include:
a. Roasting Process:
- Heating Cinnabar: The cinnabar ore (HgS) is heated in a furnace in the presence of oxygen. This oxidizes the sulfur in the cinnabar to sulfur dioxide (SO₂) and releases mercury vapor.HgS+O2→Hg+SO2
- Condensation: The mercury vapor is then condensed into liquid mercury by cooling the vapor.
b. Purification:
The extracted mercury is further purified using methods such as distillation or chemical treatment to remove impurities. Mercury is often distilled by heating it to its boiling point and condensing the vapor.
6. Interesting Facts about Mercury
- Elemental Symbol: The symbol Hg comes from the Latin name for mercury, hydrargyrum, which means “liquid silver” (Greek: hydr- “water” + argyros “silver”).
- Toxicity: Mercury is highly toxic, particularly in its vapor form and in compounds such as methylmercury. Chronic exposure can lead to neurological damage.
- Minamata Disease: One of the most infamous cases of mercury poisoning is Minamata disease, caused by industrial mercury waste released into Minamata Bay, Japan, in the mid-20th century. It caused severe neurological damage in thousands of people who consumed contaminated seafood.
- Unique Physical State: Mercury is one of only two elements (the other being bromine) that are liquid at room temperature.
- Mercury in Ancient Times: Mercury was used by ancient civilizations such as the Egyptians and the Chinese in rituals and alchemy. The Chinese believed it could grant immortality, though ironically, many emperors who ingested it died from mercury poisoning.
- Environmental Impact: Mercury pollution from industries, mining, and improper disposal of mercury-containing devices continues to pose environmental hazards, especially in aquatic ecosystems.
Mercury is a captivating yet dangerous element, known for its distinctive liquid state and a wide array of uses throughout history. Its applications range from industrial processes to medical devices, but the serious health and environmental risks associated with mercury have led to global efforts to limit in use. Understanding the properties, compounds, extraction methods, and hazards of mercury allows us to appreciate its importance and dangers in the world of science.