Explore the detailed information on DDT, BHC, and Iodoform, these three topics cover their structure preparation, properties, and uses in daily life. This Learning will be crucial for applications in agriculture, public health, and the environmental concerns associated with these compounds.
Table of Contents
ToggleDDT (Dichlorodiphenyltrichloroethane)
DDT, or Dichlorodiphenyltrichloroethane, is a synthetic chemical compound primarily used as an insecticide. It was first synthesized in 1874 by Othmar Zeidler, but its insecticidal properties were discovered much later, in 1939, by Swiss chemist Paul Hermann Müller. DDT became famous during World War II for controlling malaria and typhus among troops and civilians.
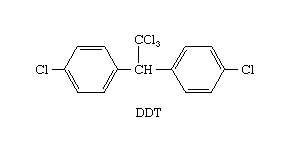
Fig: Structure of DDT
IUPAC name of DDT is 2,2-bis (p-Chlorophenyl)-1,1,1-trichloroethane) DDT.
Preparation of DDT
It is prepared by heating a mixture of choral and chlorobenzene in the molar ratio (1:2) in the presence of concentration H2SO4. DDT is prepared through a reaction known as Claisen condensation. The process involves:
- Reactants: Chloral (CCl₃CHO) and chlorobenzene (C₆H₅Cl).
- Catalyst: Sulfuric acid or oleum.
- Reaction: Chloral reacts with chlorobenzene under the influence of sulfuric acid to produce DDT.
Reaction:
CCl3CHO+2C6H5Cl H₂SO₄ →C6H4(CCl3)C(C6H4Cl)+H2O
Properties of DDT
- Physical State: DDT is a colorless, crystalline solid.
- Solubility: It is insoluble in water but soluble in organic solvents such as fats and oils.
- Stability: DDT is highly stable and resistant to breakdown in the environment, leading to its accumulation in the food chain.
Uses of DDT
- Pesticide: DDT is extensively used for the control of agricultural pests and vectors of diseases like malaria.
- Public Health: It has been employed in public health programs to reduce mosquito populations in malaria-endemic regions.
Concerns and Limitations
- Environmental Impact: DDT’s stability leads to bioaccumulation and biomagnification, causing adverse effects on wildlife and ecosystems.
- Human Health: High exposure has been linked to potential health risks such as reproductive and developmental issues. In human beings, it disturbs the sex hormones.
- Bans and Regulations: Many countries have restricted or banned DDT due to its environmental persistence and potential health risks.
BHC (Benzene Hexachloride)
BHC, or Benzene Hexachloride, refers to a mixture of isomers of hexachlorocyclohexane (HCH). The most important and active isomer is gamma-HCH, commonly known as Lindane. BHC was first synthesized in 1825 by Michael Faraday, but its insecticidal properties were discovered in 1942.
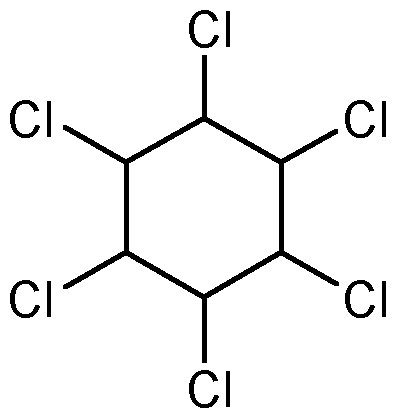
Fig: BHC Structure
IUPAC name of BHC is 1,2,3,4,5,6 Hexachlorocyclohexane (Benzene hexachloride)
Preparation of BHC
It is prepared by chlorination of benzene in the presence of sunlight.
- Reactants: Benzene (C₆H₆) and chlorine gas (Cl₂).
- Conditions: The reaction is carried out under the presence of ultraviolet light or in a dark environment to prevent the formation of polychlorinated products.
- Reaction: Chlorine reacts with benzene to produce different isomers of hexachlorocyclohexane.
Reaction: C6H6+3Cl2→C6H6Cl6
- Physical State: White crystalline solid with a mild, musty odor.
- Solubility: Slightly soluble in water but soluble in organic solvents.
- Isomers: BHC exists as a mixture of alpha, beta, gamma, delta, and epsilon isomers. The gamma isomer (Lindane) is the most effective insecticide.
Uses of BHC
- Agriculture: Used as an insecticide for crops and stored grains.
- Public Health: Lindane has been employed to treat lice and scabies in humans.
- Veterinary Medicine: Used for controlling external parasites on livestock.
Concerns and Limitations
- Environmental Persistence: BHC is persistent in the environment and can lead to bioaccumulation.
- Toxicity: Concerns have been raised about the potential health impacts on humans and wildlife due to its neurotoxic properties.
Iodoform (Triiodomethane)
Iodoform (CHI₃) is an organoiodine compound recognized for its antiseptic properties. It appears as a yellow solid and is known for its distinct medicinal odor. Iodoform was widely used in the 19th and early 20th centuries as a disinfectant and antiseptic.
Preparation of Iodoform
In the laboratory, iodoform is prepared by the action of iodine on ethyl alcohol or acetone in the presence of an alkali. The reaction is known as the iodoform reaction. Iodoform can be prepared through the Iodoform test, a reaction involving:
- Reactants: Ethanol, acetone, or other methyl ketones, iodine (I₂), and sodium hydroxide (NaOH).
- Reaction: The reaction occurs when iodine is reacted with an alcohol or ketone in the presence of a base, producing iodoform as a yellow precipitate.
General Reaction:
CH3COCH3+3I2+4NaOH→CHI3+CH3COONa+3NaI+3H2O
-
Preparation of iodoform from ethyl alcohol
2NaOH+I2→NaOI+NaI+H2O
CH3CH2OH+NaOI−→−−−−−Oxidation CH3CHO+NaI+H2O
CH3CHO+3NaOI−→−−−−−Iodination Cl3CHO+3NaOH
Where; Cl3CHO=Triiodo ethanal
Cl3CHO+NaO→CHI3+HCOONa
Where, CHI3=Iodoform
CHI3=Iodoform
HCOONa=Sodiumformate
The overall reaction may be written as;
CH3CH2OH+4I2+6NaOH−→−heatCHI3+5NaI+5H2O+HCOONa
Where; CH3CH2OH=ethyl ethanol
CHI3=Iodoform
HCOONa=Sodiumformate
2. Preparation of iodoform from acetone
Reactions involved in the preparation of iodoform from acetone are:
2NaOH+I2→NaOI+NaI+H2O
CH3COCH3+3NaOI−→−−−−−Iodination Cl3COCH3+3NaOH
Where; Cl3COCH3=Triiodo acetone
Cl3COCH3+NaOH−→−−−−−Hydrolysis CHI3+CH3CooNa
Where; CHI3=Iodoform
CH3COONa=Sodiumacetate
The overall reaction may be represented as;
CH3COCH3+3I2+4NaOH−→−heat CHI3+3NaI+CH3COONa
Where; CH3COCH3=Acetone
CHI3=Iodoform
CH3COONa=Sodiumacetate,
In the above reaction sodium carbonate can also be used in place of NaOH or KOH). If sodium carbonate is used the overall reactions are:
CH3CH2OH+4I2+3Na2CO3−→−heat CHI3+HCOONA+5NaI+2H2O+3CO2
Where; CH3CH2OH=ethanol (Ethyl alcohol)
CH3COCH3+3I2+2Na2CO3−→−heat CHI3+CH3COONa+3NaI+2Co2
Where; CH3COCH3=Propanone (Acetone)
Properties of Iodoform
Physical Properties;
- Appearance: Yellow crystalline solid with a pungent odor.
- Melting Point: Approximately 119°C.
- Solubility: Insoluble in water but soluble in ethanol, ether, and chloroform.
Chemical Properties
Some important chemical properties of Iodoform are as follows:
1. Stability.
It is not a very stable compound. On heating decomposes to give iodine vapors. The decomposition accelerates in the presence of moisture, light, and air.
4CHI3+5O2−→−−Heat 6I2+4CO2+2H2O
2. Hydrolysis.
Iodoform on boiling with alcoholic or concentrated aqueous solution of KOH gives potassium formate.
CHI3+3KOH−→−Boil HCOOH+3KI+H2O
HCOOH+KOH→HCOOK+H2O
3. Reduction.
Iodoform on reduction with red phosphorus and hydriodic acid gives diiodomethane.
CHI3+2[H]−→−−−−RedPHICH2I2+HI
4. Action with silver powder.
Iodoform oh cheating with silver power forms acetylene.
2CHI3+6Ag−→−heat CH≡CH+6AgI
Where; CH≡CH=Ethyne (Acetylene)
Uses of Iodoform
- Antiseptic: Historically used in wound dressings due to its antibacterial properties.
- Pharmaceuticals: Used in some medicinal preparations as a mild antiseptic.
- Organic Synthesis: Occasionally utilized as a reagent in the synthesis of other compounds.
Concerns and Limitations
- Odor: The strong, characteristic smell of iodoform limits its use in modern times.
- Toxicity: Prolonged exposure can lead to skin irritation and other health concerns.
Short Notes Summary
- DDT: A synthetic insecticide with high environmental persistence; used to control disease vectors and agricultural pests but banned in many countries due to ecological and health concerns.
- BHC (Lindane): An insecticide that includes several isomers; effective but associated with environmental and health risks.
- Iodoform: A yellow, antiseptic compound known for its distinct odor; formerly used in medical treatments.