Introduction to Electrochemistry
Electrochemistry is the branch of chemistry that deals with the relationship between electrical energy and chemical reactions. It involves the study of how chemical reactions can produce electrical energy and how electrical energy can induce chemical changes.
Electrolysis (strong and weak electrolyte)
The passage of an electric current through an electrolyte with subsequent migration of positively and negatively charged ions to the negative and positive electrode
| Strong electrolyte | Weak electrolyte. |
| 1. The electrolytes that ionize or dissociate almost completely into ions in aqueous solution are called a strong electrolyte. | 1. The electrolytes which ionize to a small extent in aqueous solution are called weak electrolytes. |
| 2. The solution of strong electrolyte is a good conductor of electricity and has a high value of covalent conductance even at low concentrations. Eg: Acids: HCl, H2SO4, HNO3. Bases: NaOH, KOH. | 2. Aqueous solution of weak electrolyte is a poor conductor of electricity and has a low value of equivalent conductance. Eg: Acids: H2SO3, H2CO3.
Bases: NH4OH. |
Arrhenius’s Theory of Ionization
Arrhenius’s theory of ionization consists of the following postulates.
The substances called electrolytes are believed to contain electrically charged particles called ions. These charges are positive for H+ ions or ions derived from metals and negative for the ions derived from non-metals. The number of electrical charges carried by an ion is equal to the valency of the corresponding atom.
Molecules of electrolytes (acids, bases, and salts) dissociate into oppositely charged ions on dissolution in water, e.g.
NaCl↔↔Na+ +Cl–
HCl ↔↔ H+ +Cl–
NaOH ↔↔ Na+ + OH–
The number of positive and negative charges on the ions must be equal so that the solution as a whole remains neutral.
In solution, the ions are in a state of disorderly or random motion. Upon colliding they may combine to give unionized molecules. Thus ionization is a reversible process in which the solution contains ions of electrolyte together with unionized molecules.
H2SO4(aq) ↔↔2H+(aq) + SO4-2(aq)
The extent of ionization or the degree of ionization depends upon the nature of the electrolyte. Strong electrolytes such as HCl etc. ionize completely in water. Weak electrolytes such as acetic acid (CH3COOH) ionize only slightly
Ionization is not affected by electric current.
When an electric current is passed through an electrolytic solution, charges move toward their respective electrodes, i.e. cations towards the anode and anions towards the cathode. When these ions reach their respective electrodes, they change into neutral species by the gain or loss of electrons.
The dissociation of electrolytes depends upon:
-Nature of electrolyte
-Degree of dilution
– Temperature
-The electrical conductivity depends upon :
– The number of ions present in the solution
Speed of ions
Faraday’s Laws of Electrolysis
Faraday’s first law of electrolysis
It states that the mass of the substance deposited or liberated at any electrodes during electrolysis is directly proportional to the quantity of charge passed through the electrolyte.
Mathematically,
Or, m ∝∝ Q.
It implies that,
Or, m = ZQ …(i)
Where, m = deposited mass.
Z = electrochemical equivalent.
Q = quantity of charge passed.
We have,
Q = It…(ii)Where, I = current in ampere.
t = time in seconds.
From equation (i) and (ii), we get.
Or, m = ZIT ….(iii)
Equation (iii) is the mathematical form of Faraday’s first law of electrolysis states that the mass of the substance deposited or liberated at any electrodes during electrolysis is directly proportional to the quantity of charge passed through the electrolyte.
Mathematically,
Or, m ∝∝ Q.
It implies that,
Or, m = ZQ …(i)
Where, m = deposited mass.
Z = electrochemical equivalent.
Q = quantity of charge passed.
We have,
Q = It…(ii)
Where I = current in ampere.
t = time in seconds.
From equation (i) and (ii), we get.
Or, m = ZIT ….(iii)
The equation (iii) is the mathematical form of Faraday’s first law of electrolysis
Faraday’s second law of electrolysis:
Consider three voltmeters containing dil. HCl, CuSO4 solution, and AgNO3 solution separately. Platinum (Pt) electrodes are dipped in dil. HCl solution, electrodes are dipped into the CuSO4 solution and Ag electrodes are dipped into the AgNO3 solution. These voltmeters are connected in series combination as shown in series. When the charge is passed throughout the circuit. Therefore, from Faraday’s 2nd law of electrolysis equation i.e. m = kt.
Or, m1E1=k(constant)m1E1=k(constant)
Or, m2E2=km2E2=k (constant)
And m3k3=km3k3=k (consant)
Where, m1, m2, and m3 are the masses of hydrogen and silver deposited respectively. E1, E2, and E3 are the chemical equivalents of hydrogen, copper, and silver respectively.
Or, m1E1=m2E2=m3E3=km1E1=m2E2=m3E3=k(constant) ….(ii)
The equation (ii) can be also written as:
Or, m1E1=m2E2m1E1=m2E2.
This implies that,
Or, m1m2=E1E2m1m2=E1E2….(iii)
This is the verification of the 2nd law of electrolysis
Criteria of product formation during electrolysis
Electrolysis of sodium chloride
- i) Molten sodium chloride
The only ions present are Na+ and Cl–
Anode: Cl– →Cl + e
CL(g)+ CL(g)→CL2
- ii) Concentrated aqueous sodium chloride
The products are not the same because water is also involved.
H2 O(l)à ½ O2 (g)+ 2H++ 2e– (oxidation)
2H2 O(l)+ 2e–àH2(g)+ 2 OH– (aq) (reduction)
H2O(1)→ ½ O2 (g)
Probable reactions at the cathode
Na+(aq)+ e–àNa(s)E0Red= – 2.71V
2H2 O(l)+ 2e–àH2(g)+ 2OH– (aq)E0Red= – 0.83V
The reduction potential of water is greater so H2 is evolved.
Probable reactions at the anode
2Cl–(aq)àCl2(g)+2e–E0Red= +1.36V
H2 O(l)འO2(g)+ 2H+(aq)+ 2e–E0Red= +1.23V
The reduction potentials are nearly the same.
Cl2 is preferentially evolved because of
iii) Higher concentration of Cl– ions.
- iv) Overvoltage of hydrogen. (Extra voltage is required for that reaction because it is a kinetically slow process.)
Actual reactions taking place are:
Cathode: 2H2 O(l)+ 2e–àH2(g)+ 2OH– (aq)
Anode : 2Cl– (aq) à Cl2(g)+ 2 e–
Overall reaction :2H2 O + 2Cl– à H2 (g)+ Cl2(g)+ 2OH–(aq)
- v) Electrolysis of aqueous CuSO4 using platinum electrode.
Comparing the reduction potentials, the actual reactions are
Cathode : Cu(aq)2++ 2e– à Cu(s)
Anode: H2O(l)à12\dagO2\dag+2H+(aq)\dag+2e−12\dagO2\dag+2H+(aq)\dag+2e−
- vi) Electrolysis of CuSO4using copper electrodes
The actual reactions are
Cathode :Cu2+\dag+2e−→\dagCu(s)Cu2+\dag+2e−→\dagCu(s)
Anode :Cu(s)à Cu2+ + 2e
Electrolytic conduction, equivalent, and molar conductivities
Electrolytic Conduction
Electrolytes are substances that conduct electricity due to the drifting of ions. The positive ions are called cations, and the negative ions anions. The common examples of electrolytes are aqueous solutions of inorganic salts, acids, and bases. Salts like NaCl, and KCl are electrolytes in their molten state. Solutions of organic compounds are poor conductors. Solid-state electrolytes (eg. AgI) with mobile ions are also present.
Here, we will see how the aqueous solution of common salt conducts electricity. Solid crystalline NaCl is made up of Na+ and Cl– ions bound by a strong force of attraction. The energy required to separate Na+ and Cl– ions (i.e., dissociate them) is ~7.9eV per molecule. The thermal energy at room temperature is only 0.03eV per molecule, and thus cannot dissociate NaCl. However, when NaCl is dissolved in water, the force of attraction is greatly reduced because of the high dielectric constant (= 81) of water. The force reduces by a factor of 81, and the thermal energy is sufficient to dissociate completely into Na+ and Cl– ions. This process is called ionization.
Electrolyte conductivity is smaller than that of metals by a factor of 10-5 to 10-6 at room temperature. This is due to the smaller number density of ions as compared to free electrons, the greater viscosity of the medium in which they move, and the larger mass of ions.
Specific conductance:
It is the reciprocal of specific resistance.
K =1ρ1ρ
It is the conductance of a solution of 1 cm in length having 1 square cm as an area of the cross-section.
Specific conductance depends upon the concentration of the solution.
1\dagρ1\dagρ = 1R1R* 1a1a
K = C * 1a1a
Equivalence conductance:
- Equivalent conductance(Λeq): The conductance of that volume of solution containing one equivalent of an electrolyte is known as equivalent conductance. It is denoted by Λ.
Λeq= K * V
V = Volume of solution which has one equivalent in it.
V = 1000Ncc1000Ncc
N normality (Number of equivalents per liter or 1000cc)
Λ eq= K * 1000N1000N
Units of Λ eq= K * V
=Ohm-1 cm-1cm3equivcm3equiv
= Ohm-1 cm2mol-1
Molar Conductivity (Λm):
It is defined as the conductance of 1 cm3 of volume of electrolyte which has one mole dissolved in it.
Units: Ohm-1 cm-1 or S/cm-1
Variation of conductivity with concentration
Λ m of electrolytes increases with dilution.
The variation is different for strong and weak electrolytes.
Strong Electrolytes:
It is given according to the equation
\dag\dagΛcm\dag\dagΛmc=Λ∞mΛm∞– b √ c (DebyeHuckleonsager equation )
\dag\dagΛcm\dag\dagΛmcandΛ∞mΛm∞is the molar conductance at a given concentration and at infinite dilution (respectively). b is a constant depending on the viscosity of the solvent. The graph shows that
Λ m decreases as the concentration increases.
This is because at higher concentrations there is a greater inter-ionic attraction which retards the motion of the ions as conductance falls\dag\dagΛcm\dag\dagΛmcandΛ∞mΛm∞is that conductance at infinite dilution where the ions are far apart and there is no inter-ionic attraction. This can be obtained by extrapolation of the graph to zero concentration.
Weak electrolytes:
A weak electrolyte dissociates to a much lesser extent so its conductance is lower than that of a strong electrolyte at the same concentration.
The very large increase at infinite dilution is because the ionization increases and so the number of ions in solution increases.
The value of \dag\dagΛcm\dag\dagΛmcandΛ∞mΛm∞cannot be obtained by extrapolation as can be seen on the graph. It is obtained by applying Kohlrausch’s law.
ΛmValues for strong electrolytes are larger than weak electrolytes for the same concentration. Increase \dagΛm\dagΛm for strong electrolytes is quite small as compared to that for weak electrolytes.
Electrode potential
It is important to understand the development of charges at the electrodes. When a strip of metal M is placed in a solution of its ions Mn+, a metal-metal ion electrode is obtained. The possible processes that can occur at the electrodes are:
-The metal ion Mn+ collides with the electrode and undergoes no change.
-A metal ion Mn+ collides with the electrode, gains n electrons, and gets converted into a metal atom M (i.e., the metal ion is reduced).
– A metal atom on the electrode M may lose n electrons to the electrode, and enter the solution as Mn+, (i.e., the metal atom is oxidized).
Standard electrode potential:
It is the potential developed when the pure metal is in contact with its ions at one molar concentration at a temperature of 25oC or 298 K.
Example: When a Zn rod of any length is dipped in 1M ZnSO4 solution, the standard electrode is formed and the potential developed is called standard zinc electrode potential (E ° Zn).
Fig; standard electrode potential
The standard zinc electrode is represented as Zn/Zn+2 (1M).
In the case of a gas electrode, the standard electrode potential (Eo) is defined as the potential developed at the interface of the gas and solution containing its own ions when an equilibrium is established between the gas at a pressure of 760 mm of Hg and the ions in solution of unit concentration.
When the H2 gas at a pressure of 1atm is bubbled through HCl of 1 M std H2electrode is formed and the potential developed is called standard hydrogen electrode potential (EoH2) whose magnitude is considered to be 0.
The standard H2 electrode is represented as Pt, H2 / H+(760 mm of Hg)/ (1M)
The magnitude of the standard electrode potential is independent of temperature since it depends only on the concentration of the ions.
Standard Hydrogen Electrode (S.H.E or N.H.E.)
A hydrogen electrode in which the pressure of hydrogen gas is maintained at 1 atm, and the concentration of H+ ions in the solution is 1M is called a standard hydrogen electrode (SHE). As the potential of a standard hydrogen electrode is taken as 0.00 V at all temperatures. This electrode is used as a primary reference electrode for measuring the potential of all other electrodes.
The potential of hydrogen electrode depends upon,
-Concentration of H+ ions in solution.
-Pressure of the hydrogen gas.
Thus, the potential of an electrode relative to a standard hydrogen electrode at 298 K, and 1 atm pressure when the concentration of the ion taking part in the electrode reaction is 1 mol L-1 is called the standard electrode potential.
The electrode potential of an electrode can be determined by connecting its half-cell with a standard hydrogen electrode. As the electrode potential of the standard hydrogen electrode is assigned zero, the electrode potential of the metal electrode as determined with respect to the standard or normal hydrogen electrode is called electrode potential (E).
Electrochemical series and its applications:
- An electrochemical series is a series of chemical elements arranged in order of their standard electrode potentials. The hydrogen electrode. H+(aq) + e–↔↔1/2H2(g) is taken as having zero electrode potential. An electrode potential is, by definition, a reduction potential.
Application of Electrochemical series:
-Prediction of anode and cathode:
Elements having lower reduction potential act as anode and elements having higher reduction potential act as cathode.
–To predict the displacement of elements:
Elements having lower reduction potential can displace other elements. Similarly, metals lying above the hydrogen in the electrochemical series can displace hydrogen from dilute mineral acid because they all have lower reduction potential than hydrogen.
-To calculate the emf of the cell.
Having the reduction potential of electrode emf. of the cell can be calculated by Ecell = Ecathode – Eanode.
-To predict the feasibility of a chemical cell.
A cell having positive emf is feasibility i.e.
Ecell = +ve cell is feasible and non – spontaneous.
Ecell = – ve cell is non–feasible and non- – spontaneous.
(v) To predict the free energy i.e. ΔΔG = -nFEcell
(vi) To predict the reducing and oxidizing properties:
In electrochemical series on moving from top to bottom reducing property decreases. So, the Li is the most powerful reducing agent whereas fluorine is the least powerful reducing agent. This implies elements having low reduction potential are electropositive and strong reducing agents.
Electrochemical cell
Spontaneous redox reactions are the metal displacement reactions. Therefore such reactions have been used for producing electricity. This is done by carrying out these reactions in specially designed units called electrochemical cells.
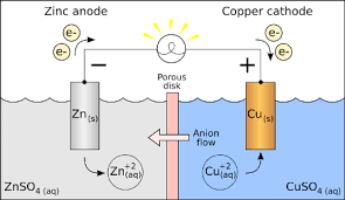
An electrochemical cell is a device used to convert the chemical energy of an indirect redox reaction into electrical energy. This is also called a Voltaic cell or a Galvanic cell. It is set up by dipping two electrodes (conducting rods) into the same or two different electrolytes. No reaction takes place inside the cell until a conducting wire joins the two electrodes.
Fig:- An electrochemical cell (a) one electrolyte (b) two electrolytes
A typical metal displacement reaction is the reaction between zinc metal and copper sulphate solution i.e.,
Zn(s)+ CuSO4(Aq)àCu+ ZnSO4(aq)
The electrochemical cell based on this reaction is set up as follows.
Zinc sulphate solution is taken in a beaker and a zinc rod is dipped in to it. Similarly, copper sulphate solution is taken in another beaker and a copper strip is dipped into it. An inverted U tube containing concentrated solutions of inert electrolytes such as KCl, KNO3, etc., connects the two solutions. The two openings of the U tube are plugged with porous materials like glass wool or cotton. This U tube is called a salt bridge as it acts like a bridge connecting the solutions of the two beakers. In place of a salt bridge, one can also use either a paper strip, unglazed porcelain clay porous pot, or asbestos fiber for developing electrical contact between the two half-cells. When a key is inserted to complete the outer circuit, the following observations are made.
-There is a flow of electrical current through the external circuit.
-The zinc rod loses weight, while the copper rod acquires weight.
-The concentration of ZnSO4 solution increases, while that of CuSO4 solution decreases.
-The two solutions in the beakers have electrical neutrality.
EMF of electrochemical cell in the standard state
The electrochemical cell consists of two half-cells where one of the half-cells has a higher value of reduction potential as compared to the other. As a result of this potential difference, there is a flow of electrons from the electrode with a lower reduction potential (or higher oxidation potential) to the electrode with a higher reduction potential (or lower oxidation potential). The difference between the electrode potentials of the two electrodes in the electrochemical cell is known as the electromotive force or cell potential of a cell. The electromotive force is commonly abbreviated as EMF (emf) and is expressed in volts. The emf of a cell may be expressed in terms of the difference in the reduction electrode potential.
EMF = Esubstance reduced – Esubstance oxidized
EMF= ER – EL or EMF= Ecathode – Eanode
Where ER and EL represent the electrode potential of the electrode on the right-hand side and on the left-hand side respectively, it depends upon the nature of the electrodes and the concentration of the solutions in the two half-cells.