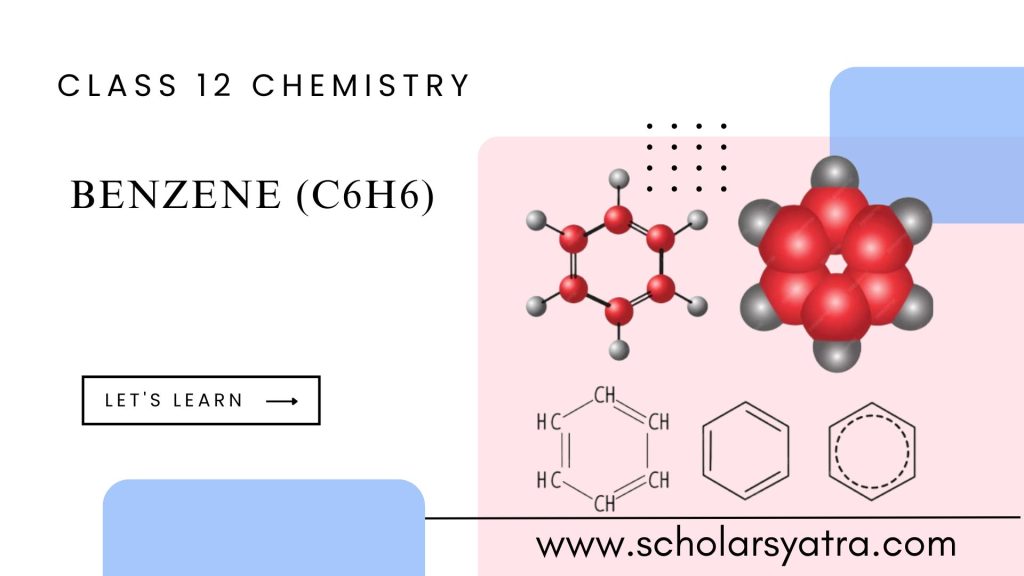
Benzene is a colorless, sweet-smelling liquid used in making plastics and other chemicals. It is highly flammable and toxic, associated with serious health risks like cancer. Let’s get started with Benzene in detail.
Table of Contents
ToggleWhat is Benzene?
Benzene is an organic compound with the formula C6H6. As one of the simplest aromatic hydrocarbons, it is composed of six carbon atoms arranged in a ring, with one hydrogen atom attached to each carbon. This unique structure makes benzene both fascinating and important in the field of organic chemistry.
The Structure of Benzene
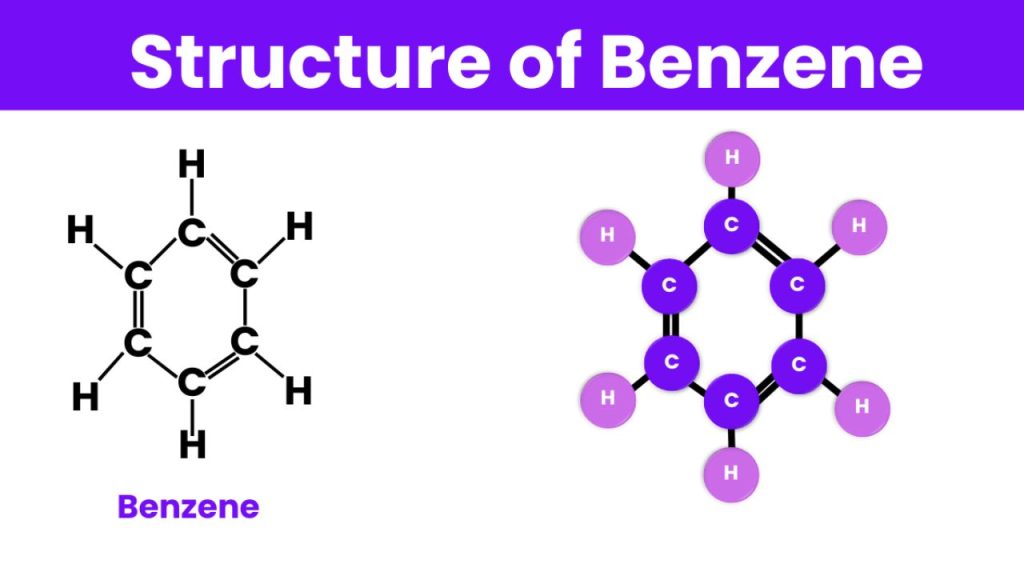
Benzene’s structure, known as the “benzene ring,” consists of six carbon atoms in a hexagonal arrangement with alternating double bonds, giving it a conjugated system. This alternating bond structure is responsible for benzene’s stability and reactivity. The resonance within the benzene ring allows for electron delocalization, which contributes to its lower reactivity compared to other unsaturated hydrocarbons.
- Molecular Formula: C6H6
- Structure: Hexagonal ring with alternating double bonds (also represented by a circle within the hexagon to denote resonance)
Kekule’S Structure of Benzene
Kekulé’s structure of benzene is one of the foundational concepts in organic chemistry that explains the structure and stability of benzene. August Kekulé, a German chemist, proposed this structure in 1865, revolutionizing the understanding of aromatic compounds. According to Kekulé, benzene’s structure consists of a six-carbon ring with alternating single and double bonds.
It has a hexagonal cyclic structure used for representing benzene in resonance hybrid structure. Every bond in the benzene ring is an intermediate of a double bond ( 1.34A0) and a single bond (1.54A0) and its bond length is 1.39A0
Every carbon atom in the benzene is sp2 hybridized. So, each carbon contains three sp2 hybrid orbitals. Two sp2 hybrid orbitals of each carbon overlap with sp2hybrid orbitals of adjacent two carbon forming ‘ C – C ‘ sigma (σ) bond. The third sp2 hybrid orbital of each carbon overlaps with the s orbital of hydrogen to form the C – H bond. Hence, there are six C – Cσ bonds and six C – Hσ bonds in benzene.
Each carbon of benzene still contains unhybridized p-orbitals. Unhybridized p orbital of each carbon laterally overlaps with each other to form π – an electron cloud above and below the plane of σ bond.
Nomenclature
The mono-substituted benzene can be named by writing the substituent as a prefix before benzene.
In disubstituted benzene, substituents are mentioned as the prefix
Isomerism
All the hydrogens in benzene are equivalent. So, it has single monosubstituted derivatives.
It gives three distributed isomers which are positional isomers of one another
Orientation in Benzene Derivatives
In electrophilic substitution, reaction in aromatic compounds, the position of the new incoming electrophile is decided by the group or atom already present in benzene. This is called orientation. This effect is called the directive effect and the groups already present in benzene are called directors or directing groups. There are two types of directing groups
i) Ortho/Para (O/P) directing group
ii) Metal (M) directing group
i) Ortho/Para (O/P) directing group: They are those types of groups in which directly bonded atom contains a lone pair of electrons. These types of atoms or groups make benzene more reactive toward electrophilic substitution reactions. Due to the presence of these groups, the incoming electrophile takes the ortho and para positions.
ii) M – directing group
Those groups in which directly bonded atoms are already bonded with more electronegative atoms by multiple bonds are called M-directing groups. They withdraw electrons from the benzene ring or aromatic system. Due to the presence of such a group, the new incoming electrophile takes a meta position with respect to the group already present in the benzene.
Note: O/P groups are ring activators. They activate the benzene ring for electrophilic substitution reaction i.e. they release electrons towards benzene and increase its electron density (except halogens). Halogens, though are O/P directors, deactivate the ring due to Negative Inductive ( -I) effect
Meta-directing groups are electron-withdrawing species and they deactivate the benzene ring for electrophilic substitution action i.e. they are ring deactivators.
Preparation of Benzene
i) Preparation of benzene from Decarboxylation: Sodium benzoate on heating with soda lime ( NaOH + CaO) gives a decarboxylated product i.e. benzene.
ii Preparation of benzene from Phenol
Vapors of Phenol, when passed through zinc dust give benzene as a reduced product.
iii) Preparation of benzene from Chlorobenzene
Chlorobenzene, on heating with a reducing agent like Lithium Aluminium Hydride ( LiAlH4), gives a reduced product as benzene.
iv) Preparation of benzene from Acetylene
When acetylene gas is passed through a red hot copper tube, it gives benzene as a polymerized product.
Properties of Benzene
Benzene is a colorless, volatile liquid with a sweet odor. It is highly flammable and slightly soluble in water, but it mixes well with organic solvents.
- Physical Properties of Benzene:
- Boiling Point: 80.1 °C
- Melting Point: 5.5 °C
- Density: 0.8765 g/cm³ at 20°C
- Chemical Properties of Benzene:
- Aromatic Stability: Due to resonance
- Reactivity: Undergoes substitution reactions rather than addition reactions typical of alkenes, preserving its aromatic ring structure
Let’s explore the Chemical Properties of Benzene with reactions:
-
Electrophilic Substitution Reaction
Benzene commonly undergoes rather than an addition reaction. It is due to the unexpected extra stability of benzene and it is a resonance hybrid of Kekule’s structures. Electrophile, being electron deficient, can easily attack benzene due to its high electron density
a) Nitration: When benzene is treated with the nitrating mixture ( conc. HNO3+ conc.H2SO4) gives nitrobenzene. The product of introducing the – NO2 group in a compound is called nitration
b) Sulphonation: Benzene, when treated with fuming sulphuric acid at room temperature gives benzene sulphonic acid
c) Friedel Crafts’ Reaction
There are following two types of Friedel Crafts reaction
i) Friedel Craft alkylation
ii) Friedel Craft acylation
i) Friedel Craft Alkylation: Benzene, when treated with an Alkyl halide in the presence of Lewis acid (AlCl3) and ether gives alkyl benzene
ii) Friedel Craft Acylation: Benzene, when treated with an acyl halide, acid halide, or acetic anhydride in the presence of Lewis acid and ether gives acyl benzene
2. Addition Reaction
a) Catalytic hydrogenation: Vapors of benzene and hydrogen, when passed over a heated catalyst like nickel, or platinum at a temperature of about 2000C give cyclohexane
b) Halogenation: Benzene undergoes an addition reaction in ordinary conditions or in the presence of sunlight with chlorine or bromine to give benzene hexachloride or benzene hexabromide respectively.
Benzene Hexachloride is used as an insecticide which is commercially called Gammexane.
c) Ozonolysis ( Addition of Ozone)
Benzene undergoes additional reaction with ozone to give benzene tri ozonide which on hydrolysis in the presence of zinc gives 3 molecules of glyoxal
-
Oxidation Reaction of Benzene
- Combustion: Benzene undergoes complete oxidation in the presence of oxygen to give carbon dioxide and water
- Catalytic oxidation: When vapor of benzene is passed through Vanadium pentoxide at a temperature of about 5000C gives Maleic acid, which on dehydration gives Maleic anhydride
What are the uses of Benzene?
Benzene has numerous applications in the industrial and chemical sectors:
- Production of Plastics and Resins: Benzene is a precursor in the manufacturing of materials like nylon, styrene (for polystyrene production), and phenol.
- Synthesis of Chemicals: It is a building block for making various chemicals, including detergents, dyes, and synthetic fibers.
- Medical and Pharmaceutical Industry: Benzene derivatives are used in the production of drugs and other medicinal compounds.
However, due to benzene’s toxic properties, its use in consumer products has diminished, and industries often employ stringent safety measures to protect workers and the environment.
Health Hazards and Safety Precautions
Benzene exposure poses serious health risks, as it is a known carcinogen. Long-term exposure can lead to blood disorders, such as leukemia and other cancers. Safety protocols are crucial for industries that use or produce benzene.
Safety Measures:
- Adequate ventilation in workplaces handling benzene
- Use of personal protective equipment (PPE), such as gloves and masks
- Regular monitoring of benzene levels in the air and in workers
Benzene remains a cornerstone of organic chemistry due to its unique structure and versatility. From pharmaceuticals to synthetic materials, benzene applications are vast, yet the importance of handling it safely cannot be overstated. Future developments may continue to explore safer alternatives and more efficient uses of this essential compound.