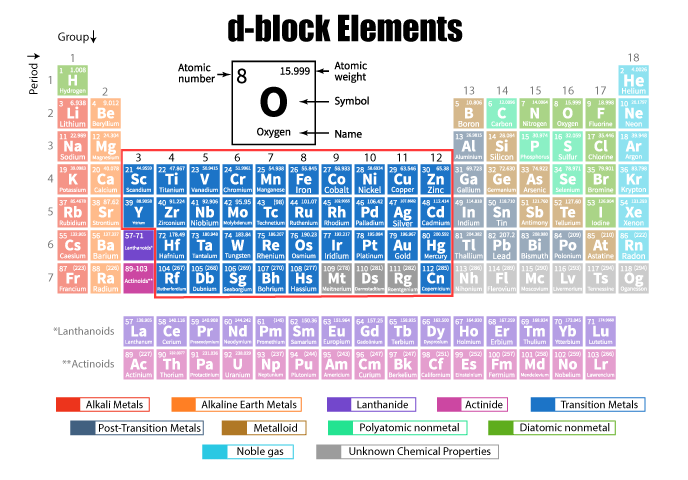
Introduction of Transition Elements
Transition elements are the group of metallic elements found in the d-block of the periodic table, specifically in groups 3 to 12. They are characterized by having partially filled d-orbitals in their atoms or ions, which leads to unique properties. These include multiple oxidation states, the ability to form colored compounds, magnetic properties, and a tendency to act as catalysts. Transition metals also form complex compounds with ligands and are known for their high melting points, mechanical strength, and the ability to form alloys. Some common examples are iron (Fe), copper (Cu), and chromium (Cr).
Table of Contents
ToggleTransition Elements
The transition elements are a group found in the middle of the periodic table, specifically in the d-block, which includes groups 3 to 12. These elements are often referred to as transition metals because they serve as a transitional bridge between the main-group elements of the periodic table, linking the metals on the left to the non-metals on the right. Transition metals have distinctive physical and chemical properties that set them apart from other elements. Let’s explore the general characteristics of transition elements in detail.
1. Electronic Configuration
The defining feature of transition elements is the gradual filling of their d-orbitals. Transition metals generally have partially filled d-orbitals, which gives rise to their unique properties. The general electronic configuration of transition elements can be represented as:
(n−1)d1−10ns0−2
- The outermost s orbital (ns) contains 1 or 2 electrons.
- As you move across the period, the (n-1)d orbitals are progressively filled with electrons.
For example:
- Scandium (Sc, Z = 21): [Ar] 3d¹ 4s²
- Iron (Fe, Z = 26): [Ar] 3d⁶ 4s²
- Copper (Cu, Z = 29): [Ar] 3d¹⁰ 4s¹
One of the unique aspects of transition metals is that the d-orbital electrons are often involved in bonding, which leads to complex and variable oxidation states.
2. Variable Oxidation States
Transition metals exhibit a variety of oxidation states due to the ability of their d-electrons to participate in bonding. Unlike main-group elements, which generally exhibit only one or two common oxidation states, transition metals can exhibit multiple oxidation states. For example:
- Iron (Fe) can exist in oxidation states of +2 (Fe²⁺) and +3 (Fe³⁺).
- Manganese (Mn) exhibits oxidation states ranging from +2 to +7.
The variability in oxidation states arises because both the ns and (n-1)d electrons can be lost during ionization. As a result, transition elements can form a wide range of compounds with different properties.
3. Formation of Colored Compounds
One of the most striking characteristics of transition metals is the formation of colored compounds. This color is due to the d-d electron transitions that occur when light is absorbed. In transition metal ions, the d-orbitals split into two energy levels due to the surrounding ligand field (crystal field splitting).
When light hits a transition metal ion, electrons can move between these energy levels, absorbing specific wavelengths of light in the process. The wavelengths of light that are not absorbed are reflected, and this determines the color that we observe. For example:
- Copper(II) sulfate (CuSO₄) appears blue.
- Potassium dichromate (K₂Cr₂O₇) appears orange.
The exact color observed depends on the oxidation state of the metal, the ligand (the molecule or ion bound to the metal), and the geometry of the complex.
4. Magnetic Properties
Transition elements exhibit interesting magnetic properties due to the presence of unpaired d-electrons. Depending on the number of unpaired electrons, transition metals can exhibit:
- Diamagnetism: No unpaired electrons are present, and the substance is weakly repelled by a magnetic field.
- Paramagnetism: One or more unpaired electrons are present, and the substance is attracted to a magnetic field.
- Ferromagnetism: In some transition metals (e.g., iron, cobalt, nickel), the unpaired electrons align in a way that results in strong, permanent magnetism.
For example, iron (Fe) in its metallic form is ferromagnetic, while many other transition metals exhibit paramagnetic behavior due to their unpaired electrons.
5. Catalytic Properties
Many transition metals are excellent catalysts in both industrial and biological reactions. Their ability to adopt multiple oxidation states and form coordination compounds makes them ideal for catalysis, as they can provide alternative reaction pathways with lower activation energies. Transition metals can act as catalysts by:
- Providing active sites for reactants to adsorb onto their surface.
- Facilitating electron transfer reactions.
- Stabilizing reaction intermediates.
Some important transition metal catalysts include:
- Iron (Fe) in the Haber process for the production of ammonia (NH₃).
- Vanadium pentoxide (V₂O₅) in the Contact process for the production of sulfuric acid (H₂SO₄).
- Platinum (Pt) and palladium (Pd) in catalytic converters for reducing vehicle emissions.
6. Tendency to Form Complex Compounds
Transition metals have a strong tendency to form complex compounds, in which the metal ion is bonded to a number of molecules or ions (called ligands) via coordinate bonds. The presence of empty d-orbitals allows transition metals to accept electron pairs from ligands, forming coordination complexes.
For example, the copper(II) ion (Cu²⁺) can form the complex ion [Cu(NH₃)₄]²⁺, in which the central copper ion is surrounded by four ammonia molecules acting as ligands.
Transition metals can form complexes with various geometries, including:
- Octahedral: Six ligands surround the metal ion, such as in [Fe(H₂O)₆]³⁺.
- Tetrahedral: Four ligands surround the metal ion, such as in [NiCl₄]²⁻.
- Square planar: Four ligands surround the metal ion in a square plane, such as in [Pt(NH₃)₂Cl₂].
The ability to form complex compounds is crucial in biological systems (e.g., hemoglobin, which contains an iron center, and chlorophyll, which contains a magnesium center).
7. High Melting and Boiling Points
Transition elements generally have high melting and boiling points due to the strong metallic bonding that arises from the delocalization of d-electrons. This leads to a high degree of cohesion between atoms, resulting in significant energy being required to break the metallic bonds. For instance:
- Iron (Fe) has a melting point of 1538°C.
- Tungsten (W) has one of the highest melting points of any element at 3422°C.
The strength of the metallic bonds also gives transition metals their characteristic hardness, durability, and high tensile strength.
8. Alloy Formation
Transition metals readily form alloys, which are mixtures of metals that exhibit superior properties compared to their component metals. The ability to form alloys is due to the similar atomic radii and crystal structures of transition metals, allowing them to replace each other in the metallic lattice.
Important alloys include:
- Steel is an alloy of iron with carbon and other elements.
- Brass is an alloy of copper and zinc.
- Bronze is an alloy of copper and tin.
Alloys are widely used in construction, manufacturing, and transportation due to their enhanced strength, resistance to corrosion, and malleability.
Why d-block elements are called transition elements?
d-block elements are called transition elements because they serve as a transition between the highly reactive metals of the s-block (groups 1 and 2) and the less reactive, non-metal elements of the p-block (groups 13 to 18) in the periodic table.
The term “transition” refers to the properties that bridge these two extremes. Specifically:
- Gradual Filling of d-Orbitals: As you move from left to right across the d-block, electrons are gradually added to the d-orbitals, resulting in a transition of chemical and physical properties.
- Variable Oxidation States: Transition elements exhibit multiple oxidation states because they can lose both their outermost s-electrons and their d-electrons, marking a change from the more fixed oxidation states of s-block metals to the diverse oxidation states of p-block elements.
- Intermediate Properties: Transition elements display intermediate properties between the highly reactive alkali and alkaline earth metals on the left, and the less reactive, more covalent, and non-metallic elements on the right.
Thus, they are called transition elements because they show a gradual shift in properties, bridging the two distinct types of elements in the periodic table.



One Response
Good 💯