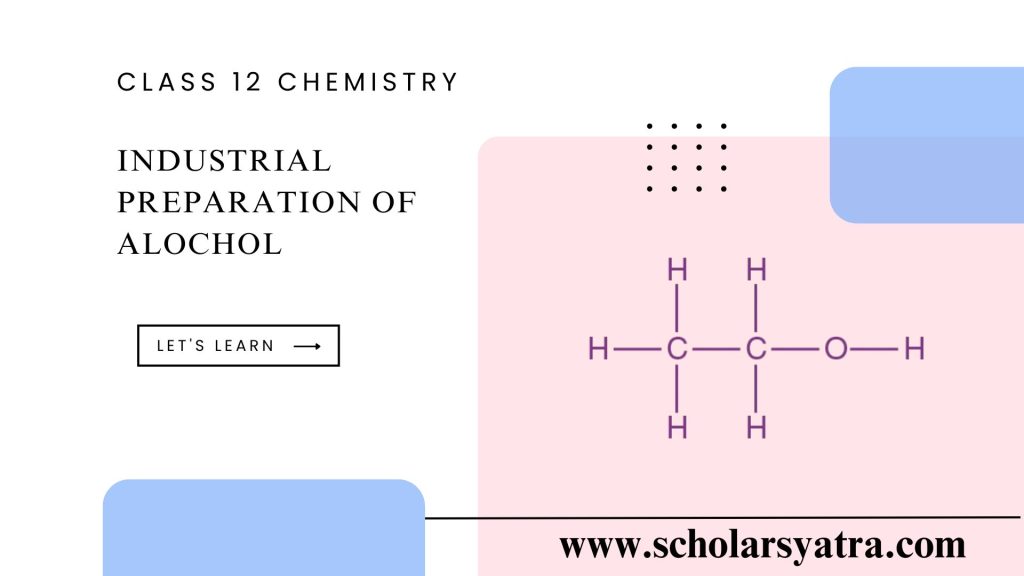
The industrial preparation of alcohol, specifically ethanol (C₂H₅OH), is widely used across various industries, including the beverage, pharmaceutical, and chemical sectors. It is carried out mainly through two processes: fermentation and synthetic methods. Both processes are chosen based on desired purity levels, production scale, and cost considerations. This article provides an in-depth overview of the main industrial methods used to produce ethanol.
Table of Contents
ToggleIndustrial Preparation of Alcohol
There are two methods to get alcohol, industrially. All these methods utilize raw materials that can be obtained from petroleum, natural gas, coal, and biomass. The methods are outlined below:
- By fermentation of carbohydrates
- By hydration of Ethylene
1. Fermentation Process
Fermentation is the most traditional method of alcohol production and involves converting sugars, primarily glucose, into ethanol and carbon dioxide by yeast under anaerobic conditions. It is a complex biochemical process that is regarded as one of the oldest methods of the manufacture of alcohol. The general reaction for fermentation is:
C6H12O6→2C2H5OH+2CO2
By this method ethyl, alcohol can be manufactured using two types of raw material molasses and starch.
Alcohol From Molasses
Molasses is a dark-colored mother liquor obtained after the crystallization of sugar. It contains carbohydrates like sucrose, glucose, fructose, etc. in significant quantity. Molasses is first hydrolyzed with water to form the dilute solution which is then mixed with yeast )a unicellular fungus containing enzymes like zymase, invertase, diastase, etc).
The alcohol obtained from molasses is called “wash” which contains a low percentage of ethyl alcohol (15-30%). When impure ethyl alcohol is subjected to fractional distillation, pure alcohol is obtained containing a purity of about 90-97%. This alcohol is called ‘rectified spirit’.
Alcohol From starch
Ethyl alcohol also is manufactured from starch-containing food grains like millet, rice, wheat, maize, buckwheat, etc. In this method, food grains are cooked at first to release starch granules. The solution is diluted with water and the resulting solution is called ‘mash’.
‘Mash’ is mixed with malt which is germinated barely containing a unicellular plant, yeast. Yeast contains different enzymes required for fermentation.
The fermented liquor obtained by this method is called ‘wash’ which is subjected to fractional distillation to obtain pure alcohol having a purity of about 90-97% and is called ‘rectified spirit’.
Major Steps in the Fermentation Process:
- Raw Material Selection:
- Common feedstocks include sugarcane, corn, and other carbohydrate-rich plants, which are rich in glucose or sucrose, and readily used by yeast for fermentation.
- Hydrolysis:
- For raw materials with complex carbohydrates (like starch or cellulose), hydrolysis is required to break down polysaccharides into simple sugars. This process uses enzymes or acid catalysts to convert polysaccharides into monosaccharides that yeast can ferment.
- Fermentation:
- Yeast, primarily Saccharomyces cerevisiae, is added to the sugar solution. The fermentation is carried out at temperatures around 30–35°C for optimal yeast activity, and the process is anaerobic (without oxygen).
- The reaction proceeds over a few days, with ethanol and carbon dioxide being the primary by-products.
- Distillation:
- After fermentation, the mixture contains ethanol along with water, unfermented sugars, and other impurities. Distillation is then employed to concentrate ethanol.
- Using a fractional distillation process, ethanol is separated at around 78.4°C (its boiling point) from water and other components, yielding around 95% ethanol purity.
- Dehydration (Optional):
- For applications that require absolute ethanol (100%), azeotropic distillation or molecular sieves are used to remove the remaining water.
Advantages of Fermentation Process
- Environmentally friendly, as it uses renewable resources.
- Generates fewer greenhouse gases compared to synthetic processes.
- Suitable for beverage, pharmaceutical, and cosmetic applications.
2. Synthetic Method: Hydration of Ethylene
The hydration of ethylene is another primary method of industrial ethanol production, primarily used in chemical industries. This process involves the catalytic addition of water to ethylene (C₂H₄) in the presence of an acid catalyst.
Reaction:
C2H4+H2O→C2H5OH
Major Steps in the Hydration Process:
- Preparation of Ethylene:
- Ethylene is produced through the steam cracking of hydrocarbons such as ethane or naphtha, common feedstocks in the petrochemical industry.
- Catalytic Hydration:
- Ethylene is mixed with steam and passed over a phosphoric acid catalyst, often supported on silica, at temperatures of 300°C and pressures of 60–70 atm.
- The reaction yields ethanol directly, although some side products can form, requiring separation.
- Purification:
- The ethanol produced is then purified by distillation to remove impurities and achieve desired purity levels.
- Ethylene hydration generally produces a highly concentrated ethanol product, which can be easily dehydrated to reach higher purity levels if needed.
Advantages of Ethylene Hydration
- More consistent and scalable compared to fermentation.
- It is ideal for chemical and industrial use where ethanol purity is paramount.
- Production can be controlled to meet high demand.
3. Comparison of Fermentation and Ethylene Hydration
| Feature | Fermentation | Ethylene Hydration |
|---|---|---|
| Feedstock | Biomass (sugarcane, corn, etc.) | Petrochemical (ethylene) |
| Purity | 95% (after distillation), requires dehydration for 100% | High purity (easily dehydrated) |
| Cost | Lower-cost feedstock but labor-intensive | Higher feedstock cost, lower labor |
| Environmental Impact | Lower emissions, renewable feedstocks | Higher emissions, nonrenewable feedstocks |
| Applications | Beverage, pharmaceutical, and industrial | Mainly industrial and chemical |
4. New Developments and Trends in Industrial Preparation of Alcohol
As the demand for sustainable fuels and chemicals grows, alternative ethanol production methods are being explored:
- Cellulosic Ethanol: Made from non-food plant materials like grasses, agricultural waste, and wood chips, cellulosic ethanol provides a renewable source without impacting food supplies.
- Gas Fermentation: Converts industrial gases like CO and CO₂ into ethanol using specialized microorganisms. This process has the potential to reduce greenhouse gases and offer a closed-loop system in industries.
- Electrochemical and Enzymatic Processes: Newer approaches use electrochemical reactions or engineered enzymes to produce ethanol more efficiently, especially at smaller scales.