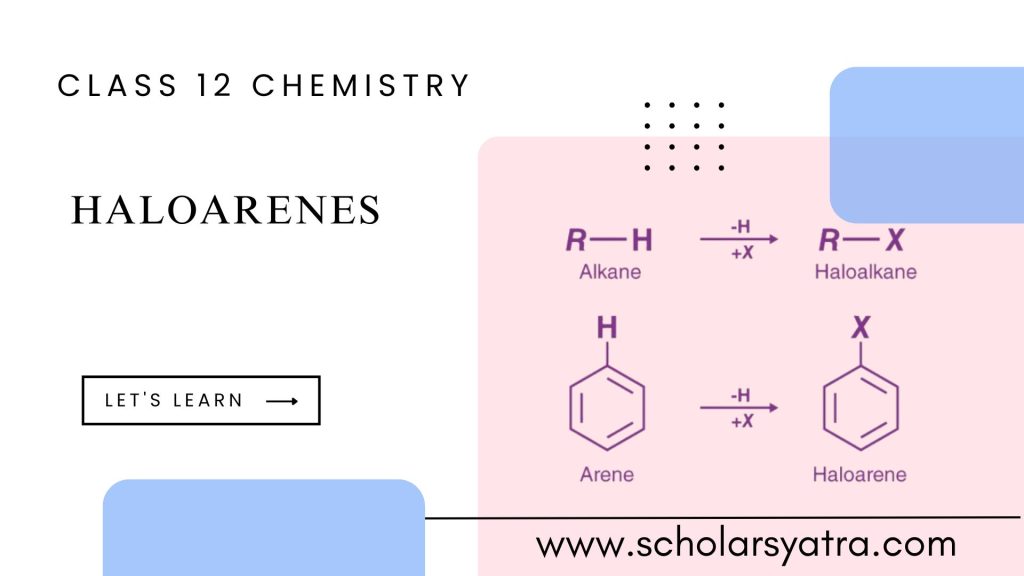
Haloarenes, also known as aryl halides, are aromatic compounds where one or more hydrogen atoms in an aromatic ring (like benzene) are replaced by halogen atoms (fluorine, chlorine, bromine, or iodine). They have diverse chemical properties and are found in various applications ranging from organic synthesis to pharmaceuticals and agrochemicals. Due to the presence of a halogen, haloarenes display distinct chemical behaviors that differ significantly from their aliphatic nature, known as alkyl halides.
Table of Contents
ToggleWhat are Haloarenes?
Haloarenes, or aryl halides, are organic compounds where a halogen atom (such as chlorine, bromine, iodine, or fluorine) is directly bonded to an aromatic ring, typically a benzene ring. In haloarenes, the carbon-halogen bond is more stable than in aliphatic halides due to the resonance structure of the aromatic ring, which delocalizes the electrons and stabilizes the molecule.
General Structure of Haloarenes:
The basic structure of a haloarene consists of an aromatic ring, such as benzene, with at least one halogen atom bonded to it. The general formula can be represented as:
Ar–X
Where:
- Ar = Aromatic ring (e.g., benzene)
- X = Halogen (F, Cl, Br, or I)
Types of Haloarenes
Haloarenes are broadly classified into two types based on the number and arrangement of halogen atoms attached to the aromatic ring:
- Monohaloarenes
- Polyhaloarenes
Let’s discuss each type in detail.
1. Monohaloarenes
In monohaloarenes, only one halogen atom is attached to an aromatic ring, typically a benzene ring.
Characteristics:
- General Formula: C6H5X where X represents a halogen (Cl, Br, I, or F).
- Structure: The halogen is directly bonded to one carbon of the benzene ring.
- Examples: Chlorobenzene (C6H5Cl), Bromobenzene (C6H5Br), and Iodobenzene (C6H5I).
- Chemical Properties:
- Reactivity: Monohaloarenes are generally less reactive towards nucleophilic substitution reactions due to the partial double bond character of the C–X bond from resonance.
- Electrophilic Substitution: They undergo electrophilic substitution reactions (like nitration, and sulfonation) on the benzene ring. The halogen is an ortho-para director, meaning: it directs incoming substituents to the ortho (adjacent) and para (opposite) positions.
- Bond Strength: The C–Cl and C–Br bonds in monohaloarenes are relatively strong due to resonance, making them more stable and harder to break compared to aliphatic halides.
Uses:
Monohaloarenes are widely used in chemical industries as intermediates in the synthesis of dyes, pharmaceuticals, and pesticides. Chlorobenzene, for instance, is used in the production of phenol and aniline.
2. Polyhaloarenes
Polyhaloarenes contain two or more halogen atoms attached to the aromatic ring. They are further classified based on the number of halogens and their positioning on the ring:
- Dihaloarenes: Two halogen atoms attached to the ring.
- Trihaloarenes: Three halogen atoms attached to the ring.
- Tetrahaloarenes: Four halogen atoms attached to the ring, and so forth.
Characteristics:
- General Formula: C6H(6−n)Xn, where n represents the number of halogens.
- Isomerism: Polyhaloarenes exhibit isomerism depending on the position of the halogens (ortho, meta, or para).
- Ortho (o-) Isomers: Halogens are attached to adjacent carbons.
- Meta (m-) Isomers: Halogens are attached to carbons with one carbon between them.
- Para (p-) Isomers: Halogens are attached to opposite carbons.
Example – Dichlorobenzene:
- 1,2-Dichlorobenzene (o-Dichlorobenzene): Halogens at adjacent carbons.
- 1,3-Dichlorobenzene (m-Dichlorobenzene): Halogens with one carbon between them.
- 1,4-Dichlorobenzene (p-Dichlorobenzene): Halogens on opposite carbons.
Chemical Properties:
- Increased Stability: More halogens enhance the resonance stabilization of the molecule but also make it more resistant to certain reactions.
- Electron-Withdrawing Effect: Halogens are electron-withdrawing, making the aromatic ring slightly deactivated toward further substitution reactions.
- Reduced Reactivity: Polyhaloarenes are generally more resistant to nucleophilic substitution reactions.
Environmental Concerns:
Some polyhaloarenes, such as polychlorinated biphenyls (PCBs), are hazardous environmental pollutants due to their persistence and bioaccumulation in ecosystems. PCBs were once used in electrical insulators and coolants but were banned in many countries due to their toxic effects.
Uses:
Polyhaloarenes are used in the synthesis of pesticides, flame retardants, and other industrial chemicals. However, due to their environmental impact, the use of certain polyhaloarenes is restricted.
Preparation of Haloarenes
Haloarenes, or aryl halides, can be prepared through several methods that involve introducing a halogen atom onto an aromatic ring. Here are the primary methods of preparing haloarenes:
1. Direct Halogenation of Aromatic Compounds
This method involves the direct addition of a halogen (like chlorine or bromine) to an aromatic compound (typically benzene) in the presence of a catalyst.
Reaction:
- Benzene reacts with chlorine or bromine in the presence of a Lewis acid catalyst like ferric chloride (FeCl₃) or ferric bromide (FeBr₃).
- This produces chlorobenzene or bromobenzene, respectively.
Example:
C6H6+Cl2FeCl3→ C6H5Cl+HCl
Notes:
- This method is used mainly for preparing chlorobenzene and bromobenzene.
- Fluorination and iodination of benzene by this method are less common due to reactivity issues.
- Fluorination is highly exothermic and can be dangerous.
- Iodination is less feasible as it requires oxidizing agents like nitric acid (HNO₃) to proceed efficiently.
2. Sandmeyer Reaction
The Sandmeyer reaction is an important method for preparing haloarenes, particularly when a specific position on the ring is required. This reaction involves converting an aromatic amine to an aryl halide.
Steps:
- Diazotization: Aniline (C₆H₅NH₂) is treated with nitrous acid (generated in situ by reacting sodium nitrite with hydrochloric acid) at low temperature to form a diazonium salt.
- Substitution: The diazonium salt is then treated with copper(I) chloride (CuCl) or copper(I) bromide (CuBr), which facilitates the replacement of the diazonium group with a halogen.
Reactions:
Formation of Benzene Diazonium Chloride:
C6H5NH2+HNO2+HCl→C6H5N2+Cl−+2H2O
Formation of Chlorobenzene:
C6H5N2+Cl− CuCl→ C6H5Cl+N2
Notes:
- This method is widely used for introducing chlorine or bromine atoms onto an aromatic ring.
- It is particularly valuable because it allows for high selectivity and control over the position of substitution.
3. Gattermann Reaction
The Gattermann reaction is similar to the Sandmeyer reaction but uses different reagents. In this reaction, a diazonium salt is prepared from aniline and then reacted with copper powder in the presence of a halogen acid (HCl or HBr).
Reaction:
C6H5N2+Cl− Cu,HCl → C6H5Cl+N2
Notes:
- The Gattermann reaction can be used to prepare both chlorobenzene and bromobenzene.
- It is less commonly used than the Sandmeyer reaction but is helpful when mild conditions are required.
4. Balz-Schiemann Reaction
The Balz-Schiemann reaction is a method specifically used for the preparation of fluoroarenes (aryl fluorides), which are challenging to produce by other means.
Steps:
- Aniline is first diazotized to form a diazonium salt.
- The diazonium salt is then treated with fluoroboric acid (HBF₄) to produce a diazonium tetrafluoroborate salt.
- This salt is then heated, decomposing it to form the fluoroarene with the release of nitrogen gas and boron trifluoride (BF₃).
Reaction:
C6H5N2+BF4− Δ→C6H5F+N2+BF3
Notes:
- The Balz-Schiemann reaction is a valuable method for preparing fluorobenzene and other aryl fluorides.
- It is limited to fluorine as a substituent and is not suitable for preparing chloro- or bromoarenes.
5. Ulmann Reaction
The Ulmann reaction is used for preparing dihaloarenes, particularly when two halogen atoms need to be introduced into the aromatic system. It is typically used to synthesize polyhalogenated aromatic compounds.
Reaction:
- In this reaction, an aryl halide reacts in the presence of copper at high temperatures to form polyhaloarenes.
Example:
C6H5I+C6H5I Cu,Δ→ C6H5−C6H5
Notes:
- The Ulmann reaction is often used for coupling reactions and synthesis of biaryls rather than for direct halogenation.
- It requires high temperatures and the presence of copper, so it is not commonly used for simple haloarene synthesis.
Physical Properties of Haloarenes
- Boiling and Melting Points: Haloarenes generally have higher boiling points than their parent aromatic compounds due to increased molecular weight and stronger intermolecular van der Waals forces.
- Solubility: Haloarenes are typically insoluble in water but are soluble in organic solvents like ether, benzene, and chloroform.
- Density: Haloarenes are denser than water due to the presence of heavy halogen atoms.
Chemical Properties of Haloarenes
Haloarenes exhibit unique chemical properties due to the influence of both the halogen and the aromatic ring.
Electrophilic Substitution Reactions
Due to resonance, the halogen is ortho- and para-directing, but its electron-withdrawing nature also deactivates the ring toward further substitution. The resonating structures of haloarene are as follows:
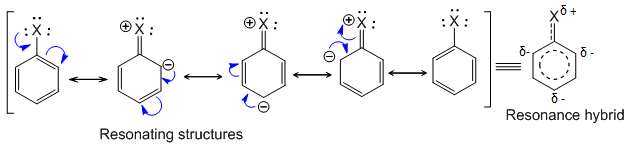
From the resonating structures, it is clear that ortho and para positions are relatively rich in electron density and hence incoming electrophile attacks at these positions give ortho and para-substituted products. Hence, haloarenes (halogens) are o/p – directing towards electrophilic substitution reaction.
Note : Halogen shows –I effect. It withdraws electrons from benzene ring and ring gets somewhat deactivated as compared to benzene.
1. Halogenation: Halogenation takes place in the presence of iron or FeCl3 or anhydrous AlCl3 as a catalyst. Eg.

2. Nitration: Eg.

3. Sulphonation: Eg.

Note : p-product is major product and o-product is minor product.
4. Friedel-Craft alkylation: eg.

5. Friedel – Craft acylation: Eg.

Nucleophilic Aromatic Substitution Reactions
Haloarenes are generally less reactive toward nucleophilic substitution than alkyl halides due to the partial double-bond character between the carbon and halogen atoms, caused by resonance.
1. Reduction of haloarenes
Haloarenes can be converted into the corresponding arenes by reduction with Ni-Al alloy in the presence of alkali.

2. Fitting And Wurtz-Fittig reaction
Haloarenes when treated with sodium in the presence of dry ether, diaryls are produced. This reaction is known as the Fitting reaction.
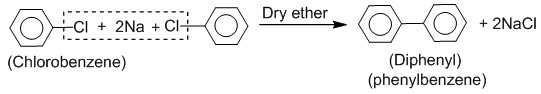
Haloarenes when treated with sodium and alkyl halide in the presence of dry ether gives toluene. This reaction is known as the Wurtz-Fittig reaction.

Some of the examples of nucleophilic substitution reactions are:
1. Reaction with aq. NaOH: When chlorobenzene is heated with an aqueous solution of NaOH at 3000C and 200 atm gives sodium phenoxide which on acidification gives phenol.

2. Reaction with aq. NH3: Chlorobenzene when heated with aq. ammonia in the presence of cuprous oxide (Cu2O) at 2000C under a pressure of 60 atm gives aniline.

3. Action with Cuprous Cyanide: Chlorobenzene when heated with cuprous cyanide (CuCN) at 2000Cin the presence of pyridine gives phenyl cyanide.

Phenyl Cyanide can be used to prepare different important compounds such as:

Applications of Haloarenes
Haloarenes are utilized in a wide range of applications due to their stability and unique chemical reactivity.
- Pharmaceuticals: Haloarenes are often used as intermediates in the synthesis of various pharmaceuticals, including antihistamines, antibiotics, and antidepressants.
- Agrochemicals: Many herbicides, insecticides, and fungicides are based on haloarene structures due to their bioactivity.
- Polymers and Synthetic Materials: Haloarenes serve as starting materials for the synthesis of various polymers, such as PVC, and in the production of dyes and pigments.
- Organic Synthesis: As intermediates in organic synthesis, haloarenes are valuable in forming carbon-carbon and carbon-heteroatom bonds.
Thank you guys! Keep Learning!!