Ethanol, or ethyl alcohol, is a simple aliphatic alcohol with the chemical formula C₂H₅OH. Ethanol (Ethyl Alcohol) is a colorless, volatile, and flammable liquid with a distinct odor and is widely used in various industrial, medicinal, and recreational applications. Due to its versatility, ethanol is one of the most important alcohols in the chemical industry and is extensively studied.
Table of Contents
ToggleEthanol (Ethyl alcohol) is produced in plants and animals by the fermentation of the carbohydrate. Thus it can be present in tissues, blood, and urine of diabetic persons. It can be prepared by any of the general methods of preparation. On the commercial scale, it is manufactured by fermentation of carbohydrates or by hydration of alkenes.
Structure of Ethyl Alcohol
Let’s understand the overview structure of Ethanol (Ethyl Alcohol):
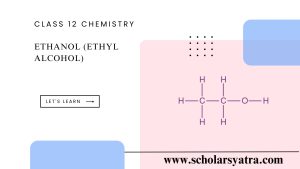
- Molecular Formula: C₂H₆O or C₂H₅OH
- Structure: Ethanol consists of a two-carbon chain with a hydroxyl group (-OH) attached to one of the carbons:
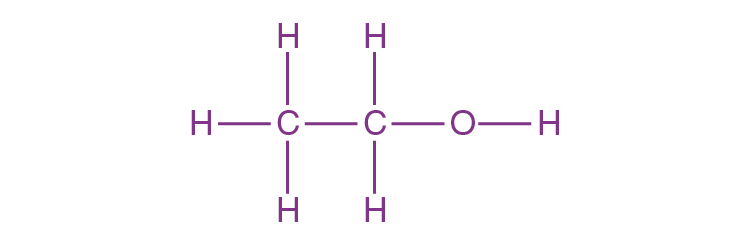
- It is a polar molecule due to the presence of the hydroxyl group, making it soluble in water.
Physical Properties of Ethanol (Ethyl Alcohol)
The physical properties of Ethanol or Ethyl Alcohol are:
- Appearance: Colorless liquid
- Odor: Characteristic alcohol odor
- Boiling Point: 78.37°C
- Melting Point: -114.1°C
- Density: 0.789 g/cm³ at 20°C
- Solubility: Miscible with water in all proportions; also soluble in most organic solvents like ether and chloroform.
- Flammability: Highly flammable; burns with a blue flame.
Chemical Properties of Ethanol (Ethyl Alcohol)
The Chemical properties of Ethanol or Ethyl Alcohol are:
- Combustion: Ethanol burns in the air to produce carbon dioxide and water:
C2H5OH+3O2→2CO2+3H2O
- Oxidation:
- Ethanol can be oxidized to acetaldehyde (C₂H₄O) using a mild oxidizing agent like pyridinium chlorochromate (PCC).
- Further oxidation produces acetic acid (CH₃COOH) with stronger oxidizing agents such as potassium dichromate (K₂Cr₂O₇) in an acidic medium.
- Reaction with Sodium: Ethanol reacts with sodium metal to produce hydrogen gas and sodium ethoxide:
2C2H5OH+2Na→2C2H5ONa+H2
- Esterification: Ethanol reacts with carboxylic acids in the presence of concentrated sulfuric acid to form esters:
C2H5OH+CH3COOH→CH3COOC2H5+H2O
Tests for Ethanol (Ethyl Alcohol)
The following tests are used for identifying ethanol (ethyl alcohol):
- Odor Test: Ethanol has a characteristic “alcoholic” smell.
- Ignition Test: Ethanol burns with a blue flame when ignited.
- Iodoform Test:
- Ethanol reacts with iodine in the presence of a base (e.g., NaOH) to produce a yellow precipitate of iodoform (CHI₃). This test confirms the presence of ethanol or a secondary alcohol with at least one methyl group attached to the carbon bearing the hydroxyl group.
- When ethanol is warmed with iodine and potassium or sodium hydroxide solution, yellow crystals of iodine are formed. This test can be employed for distinguishing ethyl alcohol from methyl alcohol as the later fails to respond to it.
- Reaction with Potassium Dichromate:
- When ethanol is heated with acidified potassium dichromate, the solution changes from orange to green due to the formation of chromium(III) ions.
- Esterification test
- Ethanol on warming with anhydrous sodium acetate and concentrated H2SO4 from ethyl acetate which is easily detected by its fruity smell.
5 uses of Ethanol (Ethyl Alcohol)
The five uses of Ethanol (Ethyl Alcohol) are listed below:
- In the Beverage Industry:
- Ethanol is the primary alcohol in alcoholic beverages, such as beer, wine, and spirits.
- As a Solvent:
- Used in the production of perfumes, varnishes, paints, and cosmetics due to its ability to dissolve many organic substances.
- In Medicine:
- Acts as a disinfectant and antiseptic.
- Used as a solvent for pharmaceuticals and as a preservative for biological specimens.
- In the Chemical Industry:
- Ethanol is used as a starting material for synthesizing various chemicals, such as ethyl acetate and diethyl ether.
- As a Fuel and Biofuel:
- Ethanol is blended with gasoline to produce gasohol, a cleaner-burning fuel.
Safety and Environmental Impact of Ethanol (Ethyl Alcohol)
- Toxicity:
- Ethanol (ethyl alcohol) is toxic in high doses, causing alcohol poisoning if ingested excessively.
- Chronic exposure may lead to liver damage and other health issues.
- Flammability:
- Being highly flammable, ethanol should be handled carefully, especially near open flames or heat sources.
- Environmental Impact:
- Ethanol is biodegradable and considered environmentally friendly as a biofuel. However, large-scale production may impact land use and water resources.
Some Modified Forms of Ethyl Alcohol
a. Industrial alcohol. The alcohol obtained from the fractional distillation of the fermented liquor contains about 95% ethanol and is called industrial alcohol. It is also known as rectified spirit and is used for industrial purposes.
b. Absolute alcohol.
Rectified spirit obtained from the distillation of the fermentation liquor wash contains about 95% of ethanol. Since a mixture of 95.6% alcohol with water boils at a lower temperature (78.10C) than the boiling point of pure alcohol (78.5%C), it is impossible to get an alcohol of higher concentration by fractional distillation of rectified spirit. Absolute alcohol (100% pure alcohol) can be obtained by digestion of rectified spirit over quick lime for several days and then distilled. The first and the last running are rejected, and the main portion of the distilled is 100% or absolute alcohol.
A modern process is the azeotropic distillation of rectified spirit with benzene. When distillation is carried out after the abduction of a certain amount of benzenes, at the first ternary mixture of water, ethanol, and benzene comes over at 650C till all the water is removed. Then the boiling point rises and the remaining benzene comes over as the binary mixture with ethanol at 600C. Finally, absolute alcohol distills at 78.50C.
c. Denature of alcohol.
The manufacture and sale of ethyl alcohol are government-controlled. Heavy excise duty is levied on the sale of alcoholic beverages. For industrial purposes alcohol is duty-free. Therefore, industrial alcohol is denatured (made for drinking purposes), by the addition of poisonous substances like methanol, acetone, or pyridine. A common practice is to add about 4 % impure methanol together with traces of pyridine and some coloring matter. The product is often sold in the maker under the name methylated spirit. Methylated spirit is extensively used for the preparation of varnishes and tinctures for external use.
d. Power alcohol.
In non-poisonous countries, alcohol mixed with petrol and benzene is now used as motor fuel. Alcohol thus used for the generation of power is popularly known as power alcohol. Rectified spirit alone does not mix with petrol, hence the third ingredient such as ether or benzene is needed. In countries like Nepal with no petroleum resources, the use of power alcohol is a dire necessity. we can make large quantities of cheap alcohol from molasses which could be used to prepare power alcohol.
e. Alcoholic beverages.
When alcohol is taken internally in small quantities, it stimulates the human system without any apparent injurious effect. However, its continuous use leads to immoderation and induces other vices. Already the governments of the various countries have schemes of prohibition in hand. Anyhow, the fact remains that alcohol is consumed in large quantities as alcoholic liquor or beverages.
Alcoholic beverages are of two types.
1. Undistilled beverages.
These are produced simply by the fermentation process from fruit juice or grains. The beverages prepared from grape juice or other juices are called wines. Wines containing 18-20 % alcohol and produced by natural fermentation are called natural wines. Weak natural; wines are made strongly by adding pure alcohol from outside and are named fortified wines.
2. Distilled beverages.
If the fermented are distilled, most of the alcohol along with other volatile produce viz, flavors, essential oils, ester, and higher alcohol pass over as distillate. The distilled liquor has a high alcohol content which may go up to 505 or even more.
That’s all for today! Keep Learning