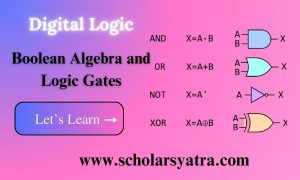
Introduction to Copper Compounds
Copper (Cu) is a transition metal that plays a significant role in both chemistry and industry. It is commonly found in nature in minerals and is known for its excellent electrical conductivity and malleability. Copper forms a variety of compounds that are widely used in various applications, from electronics to pigments. In this lesson, we will explore the different oxidation states of copper and the common compounds of copper and understand their properties, preparation, and uses.
Table of Contents
ToggleOxidation States of Copper
Copper primarily exists in two oxidation states:
- Cuprous (Copper(I), Cu⁺)
- Cupric (Copper(II), Cu²⁺)
These two oxidation states lead to different types of compounds with distinct properties.
Common Copper Compounds
1. Copper(I) Oxide/cuprous oxide (Cu₂O)
The synonyms of cuprous oxide are:
- dicopper oxide
- copper(I) oxide
- copper oxide
- cuprous oxide
- dicopper oxide
- red copper oxide
The oxidation number of copper in dicopper oxide is 1.
- Formula: Cu2O
- Hill system formula: Cu2O1
- Formula weight: 143.091
- Colour: yellow, red, or brown
- Appearance: crystalline solid
- Melting point: 1230°C
- Boiling point: 1800°C
- Density: 6000 kg m-3
| Element percentages in dicopper oxide | |
| Element | % |
| Cu | 88.82 |
| O | 11.18 |
- Appearance: Red or reddish-brown solid.
Physical properties
- It is a red-colored amorphous solid.
- It is also insoluble in water.
Chemical properties
- Action with heat: it changes to cupric oxide on heating in the presence of air.
Cu2O + O2 → CuO
- Action with acid:
Cu2O + HCl → CuCl2 + H2O +Cu (concentrated HCl)
Cu2O + H2SO4 → CuSO4 + H2O + Cu (concentrated sulphuric acid)
Cu2O + HNO3 → Cu(NO3)2 + H2O + Cu (concentrated nitric acid)
-
Preparation:
-
- Cuprous oxide is obtained by heating copper in the presence of air above 1100.
Cu + O2 → Cu2O
-
- It is also prepared by treating cupric oxide with glucose.
CuO + C6H12O6 → Cu2O + C6H17O7
- Uses
- Used in paints and pigments (red color).
- Acts as a fungicide and antifouling agent for marine environments.
2. Copper(II) Oxide/ Cupric Oxide (CuO)
The synonyms of black oxide are:
- copper oxide
- copper(II) oxide
- cupric oxide
The oxidation number of copper in the copper oxide is 2.
- Formula: CuO
- Hill system formula: Cu1O1
- Formula weight: 79.545
- Class: oxide
- Color: black or brown-black
- Appearance: crystalline solid
- Melting point: l336°C (under 1-atmosphere oxygen)
| Element percentages in copper oxide | |
| Element | % |
| Cu | 79.89 |
| O | 20.11 |
- Appearance: Black solid.
Physical properties
- It is an amorphous black oxide.
- It is insoluble in water.
Chemical properties
- Action with heat: when the cupric oxide is heated at 1000, it decomposes to cuprous oxide (Cu2O).
4CuO → 2Cu2O + O2
- Action with HCl: when the cupric oxide is treated with HCl cupric chloride is obtained.
CuO + HCl → CuCl2 + H2O
- Action with hydrogen (H2): CuO acts as an oxidizing agent.
CuO + H2 → Cu + H2O
-
Preparation:
- It is prepared by heating copper in the presence of air below 1100
2Cu + O2 → 2CuO
- It is also prepared by heating CuCO3, Cu(OH)2, CuSO4
CuCO3 → CuO +CO2
Cu(OH)2 → CuO + H2O
CuSO4 → CuO + SO2
- Uses:
- Used as a precursor to copper(II) salts.
- Widely used in ceramics, glass manufacturing, and as a catalyst in chemical reactions.
3. Copper(I) Chloride (CuCl)
- Appearance: White to light green solid.
- Properties:
- Insoluble in water but dissolves in concentrated hydrochloric acid to form complexes.
- Reacts with ammonia to form soluble complexes.
- Preparation:
- Can be synthesized by reducing copper(II) chloride.
- CuCl2+Cu→2CuCl
- Uses:
- Employed in organic synthesis as a reagent.
- Used in certain fungicides and as a component in catalytic processes.
4. Copper(II) Chloride (CuCl₂)
- Appearance: Greenish-blue crystalline solid.
- Properties:
- Highly soluble in water, producing a blue solution.
- Absorbs moisture from the air, forming a hydrate.
- Preparation:
- Formed by dissolving copper(II) oxide in hydrochloric acid.
- CuO+2HCl→CuCl2+H2O
- Uses:
- Used in dyeing and printing textiles.
- Utilized as a catalyst in organic and inorganic reactions.
5. Copper(II) Sulfate (CuSO4.5H2O)
The synonyms of blue vitriol are:
- copper sulphate pentahydrate
- copper(II) sulphate 5-water
- Roman vitriol
- Salzburg vitriol
- blue vitriol
- copper sulfate 5-water
- copper sulfate pentahydrate
- copper sulphate 5-water
- copper(II) sulfate 5-water
- cupric sulfate 5-water
- cupric sulphate 5-water
The oxidation number of copper in copper sulphate pentahydrate is 2.
- Formula: CuSO4.5H2O
- Hill system formula: Cu1H10O9S1
- Formula weight: 249.686
- Color: blue, greenish blue
- Melting point: 30°C and 110°C (dehydrates)
- Density: 2286 kg m-3
| Element percentages in copper sulfate pentahydrate | |
| Element | % |
| Cu | 25.45 |
| H | 4.04 |
| O | 57.67 |
| S | 12.84 |
- Appearance: Blue crystalline solid (pentahydrate form).
Physical properties
- It is crystalline and contains five molecules of water of crystallization.
- It is soluble in water.
-
Its aqueous solution is acidic due to hydrolysis.
Chemical properties
- Action with heat: blue vitriol on heating loses its water of crystallization and finally turns into black oxide of copper.
CuSO4∙5H2O → CuSO4∙H2O → CuSO4 → CuO + SO3
Blue vitriol at 100 loses four water of crystallizations to form the bluish-white compound and further when heated above 100 loses another water of crystallization to form white copper sulphate and when further heated at 750 forms a black oxide of copper.
- Action with ammonia solution: ammonia solution reacts with blue vitriol to form bluish-white cupric hydroxide and with excess ammonia, tetraamine copper sulphate is formed which is deep blue in color.
CuSO4 + NH4OH → Cu(OH)2 + (NH4)SO4
Cu(OH)2 + NH4OH + (NH4)SO4 → {Cu(NH3)4}SO4 + 4H2O
- Action with KI: When copper sulphate combines with potassium iodide, a violet-colored ppt. of cuprous iodide and iodine is formed.
2CuSO4 + 4KI → Cu2I2 + 2I2 + 2K2SO4
- Action with potassium ferrocyanide: The copper sulphate solution reacts with potassium ferrocyanide solution to give chocolate brown ppt of cupric ferrocyanide.
CuSO4 + K4{Fe(CN)6} → Cu2{Fe(CN)6} + 2K2SO4
- Reaction with H2S: On passing H2S gas in copper sulphate solution black ppt. of cupric sulphide is obtained.
CuSO4 + H2S → CuS + H2SO4
- Preparation:
- It is prepared by treating copper turnings with dilute sulphuric acid (H2SO4) in the presence of air.
2Cu + 2H2SO4 + O2 → 2CuSO4 + 2H2O
- It is also prepared by treating CuO, Cu(OH)2, and CuCO3 with dilute sulphuric acid (H2SO4).
CuO + H2SO4 → CusO4 + H2O
CuCO3 + H2SO4 → CuSO4 + CO2
The blue solution of copper sulphate after crystallization gives crystal of blue vitriol.
CuSO4 + 5H2O → CuSO4.5H2O
- Uses:
- Extensively used as a fungicide, algicide, and herbicide.
- Employed in electroplating and as an electrolyte in copper refining.
- Used in electroplating, and electrorefining of metal.
- Used as weedicide in swimming pool, and water reservoir tank.
- Used as the laboratory reagent.
Tests for Copper Compounds
Copper compounds exhibit characteristic tests that can help in their identification:
- Flame Test:
- Copper compounds impart a blue-green color to a flame, a test often used in the identification of copper salts.
- Formation of Blue Complex with Ammonia:
- Copper(II) compounds, when treated with ammonia, form a deep blue complex ion,[Cu(NH3)4]2+, which can be used as a confirmatory test.
Industrial and Environmental Relevance
Copper compounds have a wide range of applications, including:
- Copper Sulfate is used in agriculture as a pesticide and in industry for electroplating.
- Copper Oxides are employed in electronics, catalysis, and as pigments.
- Copper Chlorides are valuable in organic synthesis and act as catalysts in numerous reactions.
However, copper compounds must be handled with care due to their toxicity at higher concentrations, especially in aquatic environments where copper ions can disrupt ecosystems.
Summary
Copper compounds are integral to various chemical, industrial, and biological processes. The two common oxidation states, Cu(I) and Cu(II), give rise to compounds with distinct physical and chemical properties. Their wide application across industries makes understanding their behavior essential for both academic study and practical uses.
By mastering copper compounds, students will understand how transition metals behave in chemical reactions and how their compounds contribute to both natural and industrial processes.