Chloroform is an important chemical compound that has been used for various purposes, such as a solvent and formerly as an anesthetic. Its chemical behavior, history, and applications make it a significant substance in both industrial and scientific settings.
Table of Contents
ToggleWhat is Chloroform?
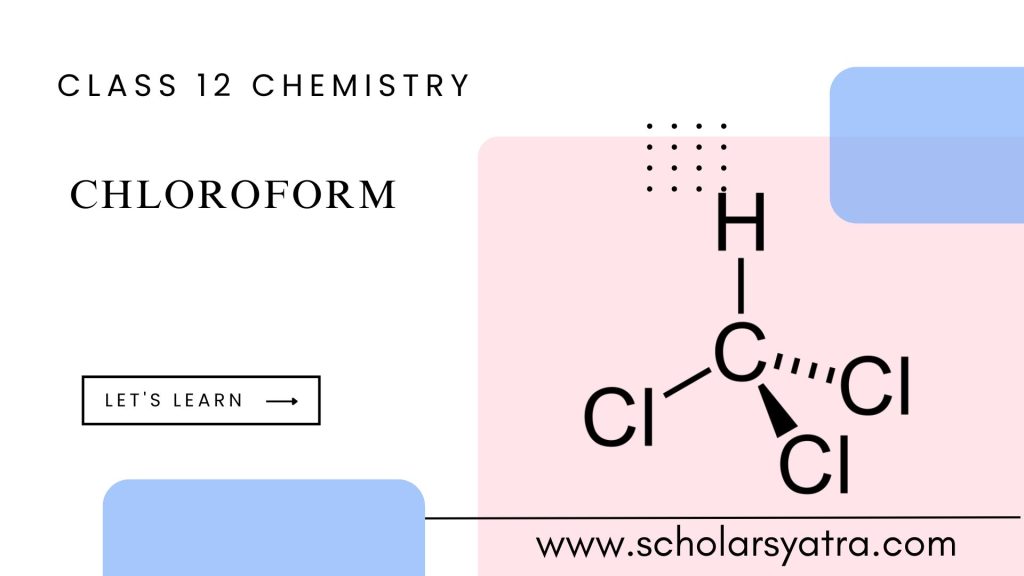
Chloroform, also known as trichloromethane, is an organic compound with a sweet-smelling characteristic. It belongs to the group of compounds known as haloalkanes and is commonly found as a colorless, dense liquid at room temperature. Generally, it is widely used as an anesthetic during surgeries. Later, phased out due to safety concerns.
Chloroform Formula
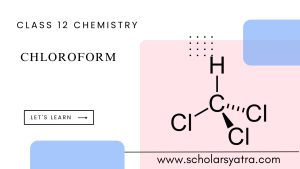
Chloroform’s molecular formula is CHCl₃, indicating that it consists of one carbon atom, one hydrogen atom, and three chlorine atoms.
How to make Chloroform
Chloroform can be prepared by various methods, including chemical reactions and through natural processes. Here, we will cover the preparation of chloroform from several chemicals.
Chloroform is prepared by heating ethanol or acetone with aqueous bleaching powder paste. Bleaching powder paste acts as an oxidizing, chlorinating, and hydrolyzing agent.

Preparation of Chloroform from Ethanol
Step I: Oxidation :

Step II: Chlorination :

Step III: Hydrolysis

Preparation of Chloroform from Acetone (propanone)
Step I: Chlorination

Step II: Hydrolysis

Natural Occurrence: Chloroform can also be produced naturally in small amounts by some algae and plants as a byproduct of the breakdown of organic matter.
Physical Properties of Chloroform
- Chloroform is a colorless, sweet-smelling liquid.
- Its freezing point is – 630C and its boiling point is 610 C.
- It is heavier than water.
- Chloroform is slightly soluble in water but partially soluble in ether, alcohol, etc.
- As inhaling the vapors of chloroform induces unconsciousness therefore it can be used as an anesthetic agent for surgery.
Chemical Properties of Chloroform
The chemical properties of chloroform are based on reactivity:
1. Reaction with Air
In the presence of sunlight, chloroform is oxidized by air to produce a highly poisonous gaseous compound called phosgene (carbonyl chloride).

Thus, chloroform is stored in a dark/colored bottle to prevent the oxidation of chloroform into phosgene.
A small amount of ethanol is also added into the chloroform at the time of packing because ethanol converts highly poisonous phosgene ( if formed) to a non-poisonous diethyl carbonate.

2. Reaction with aq. KOH solution:
When boiling with an aqueous KOH solution, chloroform is hydrolyzed to form potassium formate which on acidification gives formic acid.

3. Reaction with Silver Powder:
Chloroform when heated with silver powder gives acetylene(ethyne).

4. Reaction with Primary Amines (Carbylamine Reaction):
when chloroform is warmed with a primary amine in the presence of alcoholic KOH, an offensive(unpleasant) smell of carbylamines ( i.e. isocyanide) is obtained. This reaction is known as the Carbylamine reaction.

Secondary and tertiary amines do not respond to this reaction and therefore, this reaction is used as a test reaction for primary amines.
5. Reaction with Phenol (Riemer- Tiemann reaction)
When chloroform is heated with phenol and sodium hydroxide followed by hydrolysis, o-hydroxy benzaldehyde ( salicylaldehyde) is formed. This reaction is called the Riemer – Tiemann reaction.

6. Reaction with acetone(propanone):
Chloroform reacts with acetone in the presence of a base such as KOH to give Chloretone.

Chloretone is used as a sleep-inducing (hypnotic) drug.
7. Reaction with HNO3
On heating with conc. HNO3, chloroform gives chloropicrin.

Chloropicrin is used as an insecticide and tear gas.
8. Reduction Reaction
Chloroform on reduction with zinc dust and hydrochloric acid (i.e. acidic medium) gives methylene chloride (dichloromethane).

However, when reduced with zinc and water (i.e. neutral medium) gives acetylene (ethene).

Uses of Chloroform
- It is used as an anesthetic. It is now being replaced by other safe anesthetics because chloroform in some cases causes cardiac and respiratory failure.
- It is used as a laboratory reagent for testing primary amines.
- It is used for the preparation of Chloropicrin, Chloretone, salicylaldehyde, etc.
- It is used in medicines such as cough syrup.
- It is used as a preservative for biological specimens.
Safety and Environmental Concerns
A. Health Risks:
- Toxicity: Chloroform is toxic if ingested, inhaled, or absorbed through the skin. Prolonged exposure can damage the liver, kidneys, and central nervous system.
- Carcinogenicity: Classified as a potential human carcinogen. Chronic exposure is associated with an increased risk of cancer.
B. Environmental Impact:
- Chloroform contributes to pollution when released into the environment. It can evaporate into the air and, when broken down by sunlight, contribute to the formation of phosgene and other harmful compounds.
C. Safety Measures:
- When handling chloroform, it is essential to use personal protective equipment (PPE) such as gloves and masks. Chloroform should be stored in dark, airtight containers to minimize decomposition.
FAQs about Chloroform
Q.1: What is the nature of chloroform- acid or base?
Answer: The color is compared to the 2 mL blank of distilled water having a pH range of 5.0 – 7.0. If the sample solution is discolored i.e. yellow relative to the blank i.e. blue-green, the deuterated chloroform is acidic.
Q.2: How is chloroform rendering people unconscious?
Answer: Chloroform effectively can make a human unconscious by disrupting the major functioning of intercellular protein synthesis. Doctors are using a cloth mask having a constant drip of chloroform (or sometimes ether) onto it. Further, it is placed over the nose and mouth with a wireframe, to knock someone out with chloroform.
Q.3: How chloroform is affecting the human body?
Answer: The effects of chloroform on the human body are easily visible. Its local symptoms after chloroform inhalation include shortness of breath, and nose and throat irritation. Its acute inhalation can lead to systemic symptoms like agitation, nausea, and vomiting accompanied by ataxia, dizziness, and drowsiness.
Q.4: How much chloroform content is toxic to humans?
Answer: The toxic dose of chloroform is 7 – 25 mg/dL i.e. 0.59 – 2.1 m mol/L. At inhaled concentrations of strength less than 1500 ppm, the physical effects of dizziness, tiredness, and headache are reported. Also, anaesthesia occurs at a range of 1500 to 30,000 ppm. Chloroform generally irritates the respiratory tract.
Q.5: How one can prevent chloroform inhalation?
Answer: Medical people and people from some industries have to work with chloroform. So, such people have to ensure that all other people must be knowing his/her nature of work with chloroform. During the work, must avoid inhalation, and perform all operations in a certified chemical fume hood or other approved ventilated enclosure; Also avoid contact with it.
Happy Learning Scholars!