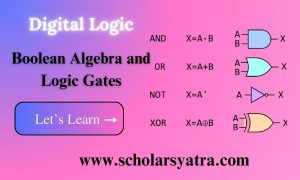
Introduction to Chemical Bonding
Chemical bonding is the force that holds atoms together in compounds and molecules. The understanding of how atoms bond and form structures is fundamental to chemistry. Atoms bond to achieve stability by fulfilling the octet rule, where they attain a full outer electron shell, typically of eight electrons. There are several types of bonds based on how the electrons are shared or transferred between atoms, which affects the physical and chemical properties of molecules.
Table of Contents
ToggleTypes of Chemical Bonds
-
Ionic Bonding
Ionic bonding occurs between atoms with significantly different electronegativities. This typically involves a metal and a non-metal. In ionic bonds:
- One atom (the metal) loses electrons to become a positively charged ion (cation).
- Another atom (the non-metal) gains those electrons to become a negatively charged ion (anion).
- The oppositely charged ions attract each other, forming a strong electrostatic bond.
Example: Sodium chloride (NaCl). Sodium (Na) loses one electron to become Na⁺, and chlorine (Cl) gains an electron to become Cl⁻. The resulting Na⁺ and Cl⁻ ions are held together by an ionic bond.
2. Covalent Bonding
Covalent bonding occurs when two atoms share electrons to fulfill the octet rule. Covalent bonds can occur between non-metals. Depending on the number of electron pairs shared, covalent bonds can be:
-
- Single Bonds: One pair of electrons is shared (e.g., H₂, where two hydrogen atoms share one pair of electrons).
- Double Bonds: Two pairs of electrons are shared (e.g., O₂, where each oxygen atom shares two pairs of electrons).
- Triple Bonds: Three pairs of electrons are shared (e.g., N₂, where nitrogen atoms share three pairs of electrons).
Covalent bonds can further be classified into:
-
- Polar Covalent Bonds: Unequal sharing of electrons due to a difference in electronegativity between the bonded atoms (e.g., H₂O, where oxygen is more electronegative than hydrogen, causing a slight charge separation).
- Non-polar Covalent Bonds: Equal sharing of electrons between atoms with similar electronegativities (e.g., Cl₂, where both chlorine atoms have the same electronegativity).
3. Metallic Bonding
Metallic bonding occurs in metals, where electrons are not shared or transferred between individual atoms but are free to move in a ‘sea of electrons’. This bonding gives metals their characteristic properties such as conductivity, malleability, and luster. The positively charged metal ions are held together by the electrostatic attraction to the sea of delocalized electrons.
Factors Influencing Bonding
- Electronegativity: The ability of an atom to attract shared electrons. Atoms with high electronegativity (such as fluorine) tend to attract electrons strongly in covalent bonds.
- Ionization Energy: The energy required to remove an electron from an atom. Higher ionization energy means the atom is less likely to lose electrons and form positive ions.
- Atomic Radius: The size of an atom affects how strongly it can attract or share electrons. Smaller atoms with more protons tend to have higher electronegativities.
Molecular Geometry and the Shape of Molecules
The shape of a molecule is crucial in determining its chemical properties and how it interacts with other molecules. The molecular shape is determined by:
- The number of bonding pairs of electrons around the central atom.
- The number of lone pairs of electrons (non-bonding electrons) on the central atom.
VSEPR Theory
VSEPR Theory (Valence Shell Electron Pair Repulsion Theory) is used to predict the geometry of molecules. According to VSEPR theory, electron pairs (bonding and lone pairs) around a central atom will arrange themselves as far apart as possible to minimize repulsion. The shape of the molecule is determined by the arrangement of these electron pairs.
Common Molecular Shapes
-
Linear Shape
Molecules with two bonding pairs and no lone pairs of electrons on the central atom form a linear shape. The bond angle is 180°.
- Example: Carbon dioxide (CO₂).
-
Trigonal Planar
When there are three bonding pairs of electrons and no lone pairs on the central atom, the molecule forms a trigonal planar shape with bond angles of 120°.
- Example: Boron trifluoride (BF₃).
-
Tetrahedral Shape
Four bonding pairs around the central atom lead to a tetrahedral shape, with bond angles of 109.5°.
- Example: Methane (CH₄).
-
Trigonal Pyramidal
A molecule with three bonding pairs and one lone pair around the central atom will form a trigonal pyramidal shape. The lone pair slightly reduces the bond angles, making them less than 109.5°.
- Example: Ammonia (NH₃).
-
Bent (or V-shaped)
When there are two bonding pairs and two lone pairs around the central atom, the molecule adopts a bent or V-shape. Lone pairs exert greater repulsion, resulting in smaller bond angles, usually around 104.5°.
- Example: Water (H₂O).
-
Trigonal Bipyramidal
Molecules with five bonding pairs form a trigonal bipyramidal shape, with bond angles of 90° and 120°.
- Example: Phosphorus pentachloride (PCl₅).
-
Octahedral Shape
Six bonding pairs around the central atom result in an octahedral shape with bond angles of 90°.
- Example: Sulfur hexafluoride (SF₆).
The main postulates of VSEPR theory are:
1. The shape of the molecule is determined by both the total number of electron pairs (bonding and non-bonding) around the molecule’s central atom and the orientation of these electron pairs in the space around the central atom.
2. To minimize the repulsion forces between them, electron pairs around the molecules’ central atom, tend to stay as far away from each other as possible
The strength of repulsions between different electron pairs follows the order:
Lone pair – Lone pair > Lone pair – Shared pair > Shared pair – Shared pair.
Prediction of molecular geometry based on VSEPR theory:
A molecule with two bond pairs:
In a molecule having two bond pairs of electrons around its central atom, the bond pairs are located on the opposite sides (at an angle of 180o) so that the repulsion between them is minimum. Such molecules are therefore linear. Some molecules, which show linear geometry are: BeF2 (beryllium fluoride), BeCl2(beryllium chloride), BeH2 (beryllium hydride), ZnCl2 (zinc chloride), and HgCl2(mercuric chloride)
Molecules with three bond pairs:
In a molecule having three bond pairs of electrons around its central atom, the electron pairs form an equilateral triangular arrangement around the central atom. These molecules have a trigonal planar (or triangular planar) shape and the three bond pairs are at 120°C to each other.
In a molecule of the type AB3, the three bond pairs of electrons are located around A in a triangular arrangement, and the molecule AB3 has a triangular planar geometry. Some molecules that show triangular planar geometry are BCl3, BF3, etc.

boron trifluoride is a trigonal planar molecule
Molecules with four bond pairs:
Molecule having four bond pairs of electrons around the central atom, arrange their electrons tetrahedrally. These molecules have tetrahedral shapes and the four bond pairs are at an angle of 109°28′ with respect to each other. Some molecules which show tetrahedral geometry are CH4, CCl4, NH4+, SiH4, etc.

Molecules with five bond pairs:
Five bond pairs orient themselves around the central atom in a trigonal bipyramidal way. A molecule having five bond pairs around its central atom has a triangular bipyramidal shape. Three bond pairs are arranged in an equatorial triangular plane and are oriented at an angle of 120° with respect to each other. The other two bond pairs are opposite to each other, and at right angles to the triangular plane formed by the three bond pairs. Some other molecules, which show trigonal bipyramidal geometry are; PCl5, PF5, and SbCl5.
For example in a molecule of type AB5, the five bond pairs are distributed in a trigonal bipyramidal around the central atom ‘A’. Therefore, the molecules of type AB5 are trigonal bipyramidal in shape.


AB5, five bond pairs are oriented around A in a trigonal bipyramidal shape. Similarly, PCl5 also has a trigonal bipyramidal shape.
Molecules with six bond pairs:
Six bond pairs in a molecule are distributed octahedrally around the central atom. A molecule having six bond pairs around its central atom has an octahedral shape. In a molecule of type AB6, the six ‘B’ atoms are placed octahedrally around ‘A’. Thus, the molecules of the type AB6 are octahedral. The molecule SF6has an octahedral geometry

AB6-type molecules are octahedral in shape. SF6 molecule has an
octahedral geometry.
Shapes of the molecules having bond pairs and lone pairs of electrons
The pairs of electrons in the valence shell of an atom, which are not involved in bonding, are called lone pairs of electrons. Well-known instances of these types of molecules are: the oxygen atom in water molecule H2O, has two lone pairs of electrons; the nitrogen atom in ammonia molecule NH3 has one lone pair of electrons. Mentioned below are a few illustrative examples of these types of molecules.
Molecules with two bond pairs and two lone pairs:
The four electron pairs (two bond pairs + two lone pairs) are distributed tetrahedrally around the central atom as shown below. The two lone pairs on the central atom repel the bond pair slightly inwards due to greater lone pair-bond pair repulsion. As a result, the bond angle in such a molecule is less than the tetrahedral value of 109°28′. The presence of only two bonds in the molecules gives a bent (V-shaped) structure.
Examples include H2S, H2O, F2O and SCl2.
H2S: Bent (V-shaped) structure. S has 6 electrons in its outermost shell. 2H-atoms contribute 2 electrons during bonding. Thus, there are 8 electrons or 4 electron pairs around S. This gives a tetrahedral distribution of electron pairs around S. The two corners of the tetrahedron are occupied by H-atoms and the other two by the lone pairs of electrons. Thus, H2S is a bent structure molecule

Molecules with four bond pairs and two lone pairs:
The four bond pairs are distributed in a planar distribution. The two lone pairs are in a direction at right angles to this plane. This gives a square planar shape to such molecules.
Examples include ICl–4, XeF4, and [Ni(CN) 4]2.

Hybridization and Molecular Orbitals
Hybridization is a concept that explains the mixing of atomic orbitals to form new hybrid orbitals. These hybrid orbitals can overlap to form bonds in molecules. Depending on the types of orbitals involved, different types of hybridization occur:
- sp Hybridization: Linear molecules with two regions of electron density (e.g., BeCl₂).
- sp² Hybridization: Trigonal planar molecules with three regions of electron density (e.g., BF₃).
- sp³ Hybridization: Tetrahedral molecules with four regions of electron density (e.g., CH₄).
The overlap of hybridized orbitals leads to the formation of sigma (σ) bonds, which are single covalent bonds. Pi (π) bonds form when unhybridized p-orbitals overlap sideways, contributing to double and triple bonds. For e.g., in the case of carbon, the ground state electronic configuration is 1s2 2s2 2p1x 1y
To explain the tetravalency of carbon, it was proposed that one of the electrons from the 2s filled orbital is promoted to the 2p empty orbital (2pz), which is in a higher energy state. Thus, four half-filled orbitals form in the valence shell this accounts for the bonding capacity of four carbon atoms. This state is known as the excited state and the configuration of carbon in the excited state is:

The above configuration reveals that all the four bonds formed by carbon will not be identical. E.g., in the formation of a CH4 molecule, one C-H bond will be formed by the overlapping of the 2s-orbital of C and 1s-orbital of H whereas the other three C-H bonds will be formed by the overlapping of 2p-orbitals of C, and 1s-orbital of H. Therefore, all the bonds will not be equivalent.
Hybridization may be defined as the phenomenon of intermixing the orbitals of slightly different energies to redistribute their energies and give a new set of orbitals of equivalent energy and shape. The new orbitals formed as a result of hybridization are called hybrid or hybridized orbitals. Thus, to form four equivalent bonds, one 2s and three 2p-orbitals of carbon hybridize and form four new orbitals. Such orbitals are called sp3 hybrid orbitals.
The important characteristics of hybridization are listed below:
(i) The number of hybridized orbitals formed is equal to the number of orbitals that get hybridized.
(ii) The hybridized orbitals are always equivalent in energy and shape.
(iii) The hybrid orbitals are more effective in forming stable bonds than the pure atomic orbitals.
(iv)The hybrid orbitals are directed in space in some preferred directions to have stable arrangements.
Therefore, the type of hybridization gives the geometry of the molecule. Depending upon the different combinations of s- and three p-orbitals, three types of hybridizations are known.
sp hybridization:
This involves the mixing of one s- and one p-orbital forming two sp-hybrid orbitals. The two sp-hybrid orbitals are oriented in a linear arrangement and the bond angle is 180°. For e.g., BeF2 involves sp-hybridization
sp2 hybridization:
In this case, one s- and two p-orbitals hybridize to form three sp2 hybrid orbitals. These three sp2 hybrid orbitals are oriented in a trigonal planar arrangement. For e.g., in BH3 boron atom undergoes sp2hybridization and therefore, BH3 has trigonal planar geometry and the HBH bond angle is 120o.
sp3 hybridization:
In this case, one s- and three p-orbitals hybridize to form four sp3 hybrid orbitals. These four sp3-hybrid orbitals are oriented in a tetrahedral arrangement. A common example of a molecule involving sp3-hybridization is methane (CH4). Therefore, CH4 has tetrahedral geometry and the HCH bond angle is 109.5o.
(i) sp hybridization

(ii) sp2 hybridization

(iii) sp3 hybridization

CONCEPT OF SIGMA BOND AND PI BOND
Sigma Bond (s)
When the overlap of the orbital of two atoms takes place along the line joining the two nuclei (orbital axis) then the covalent bond formed is called a sigma (s) bond. These bonds can be formed due to’s-s’, ‘s-p’, or ‘p-p’ overlapping along the orbital axis. Free rotation around a sigma bond is always possible.


Fig: (iv)-
Formation of sigma bond due to various overlapping Pi bonds (p)
When the two atoms overlap due to the sideways overlap of their ‘p’ orbitals, the covalent bond is called a pi(p) bond. In a pi bond, the electron density is concentrated in the region perpendicular to the bond axis.
Characteristics of the pi (p) bonds
-The pi bonds are weak because the orbital overlap is partial.
-For a complete sideways overlap the ‘p’ orbitals should be parallel to each other. This is possible when all the atoms of the molecule are in the same plane i.e., there is no rotation of one part of the molecule relative to the other about the pi (p) bond.

Fig: (v) Rotation about a double bond
In molecules containing a double bond, which has a pi bond, there is no free rotation and such molecules exist in isomeric forms of ‘cis’ and ‘trans’. In the ‘cis’ form the similar atoms lie on the same side of a plane placed along the internuclear axis while in the ‘trans’ form the similar atoms lie on the opposite sides.
The electrons in the pi (p) bond are placed above and below the plane of the bonding atoms so they are more exposed. They are more susceptible to attack by electron-seeking or oxidizing agents. Hence, they are the most reactive centers in unsaturated (multiple-bonded) compounds.
Comparative properties of sigma and pi bonds
|
Sigma (σ) bond |
Pi (π) bond |
|---|---|
| Formed due to the axial overlap of two orbitals (‘s-s’, ‘s-p’or’p-p’). | Formed by the lateral (sideways) overlap of two ‘p’ orbitals. |
| Only one sigma bond exists between two atoms. | There can be more than one pi bond between the two atoms. |
| The electron density is maximum and cylindrically symmetrical about the bond axis. | The electron density is high along the direction at right angles to the bond axis. |
| Free rotation about the sigma bond is possible. | Free rotation about the pi bond is not possible. |
| This bond can be independently formed, i.e., without the formation of a pi bond. | The pi bond is formed after the sigma bond has been formed, |
| The Sigma bond is relatively strong. | The pi bond is weak. |
Polarity of Molecules
The shape of a molecule and the type of bonding affect its polarity. Polar molecules have a dipole moment due to an uneven distribution of electron density. Non-polar molecules, on the other hand, have a symmetrical electron distribution.
Key Factors Influencing Molecular Polarity:
- Bond Polarity: Determined by the difference in electronegativity between bonded atoms.
- Molecular Shape: Even if a molecule contains polar bonds, it can still be non-polar if the shape is symmetrical, canceling out the dipole moments.
Example:
- Water (H₂O) is polar due to its bent shape and the difference in electronegativity between oxygen and hydrogen.
- Carbon dioxide (CO₂), although containing polar bonds, is non-polar because of its linear symmetry.
Understanding chemical bonding and the shape of molecules is crucial in predicting the behavior, reactivity, and properties of substances. Ionic, covalent, and metallic bonds create the framework for chemical compounds, while molecular shape and polarity influence how these compounds interact with each other. Using theories such as VSEPR and hybridization allows chemists to predict molecular structures and properties accurately, providing insight into the complexities of chemical interactions.