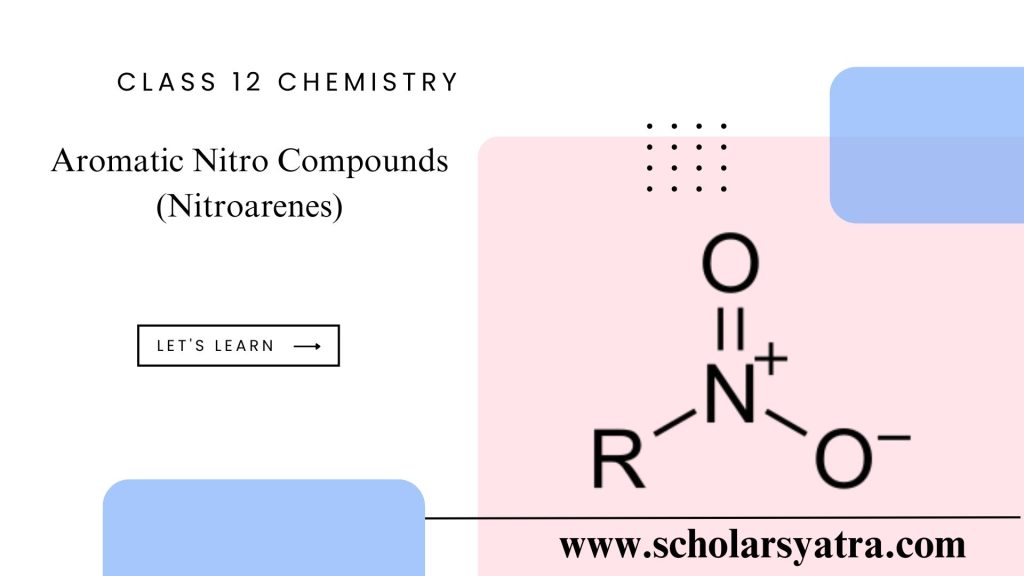
Compounds in which the nitro group ( -NO2) is directly bonded to the benzene (aromatic) ring are called aromatic nitro compounds or simply nitroarenes. These are also regarded as nitro derivatives of arenas.
Table of Contents
ToggleIntroduction to Aromatic Nitro Compounds
Aromatic nitro compounds are a significant class of organic molecules characterized by one or more nitro groups (-NO₂) attached to an aromatic ring. These compounds are vital in industrial chemistry, serving as intermediates in the synthesis of dyes, pharmaceuticals, and explosives.
Aromatic nitro compounds Examples
- Nitrobenzene (C₆H₅NO₂) – A pale yellow liquid with an almond-like odor, used in aniline production.
- Dinitrotoluene and Trinitrotoluene (TNT) are known for their applications in explosives.
The nitro group introduces unique chemical properties, including strong electron-withdrawing behavior, which influences the reactivity and stability of these compounds.
Structure of Nitro Compounds
Structure of the Nitro Group:
- The nitro group (-NO₂) consists of one nitrogen atom bonded to two oxygen atoms through one double bond and one coordinate bond.
- The nitrogen atom is sp² hybridized, giving the nitro group a planar structure.
- Resonance stabilization:
R−NO2↔R−N+=O−O−
Electron-Withdrawing Nature:
- The nitro group strongly pulls electron density away from the aromatic ring due to its resonance and inductive effects.
- This influences the ring’s reactivity, making it less reactive toward electrophilic substitution.
Methods of Preparation of Aromatic Nitro Compounds
Preparation of nitroaromatics are
Nitration Reaction:
- Reaction: Aromatic compounds react with a nitrating mixture (concentrated HNO₃ and H₂SO₄).
- Mechanism:
- Formation of the nitronium ion (NO₂⁺) from HNO₃ and H₂SO₄.
- Electrophilic attack of NO₂⁺ on the aromatic ring.
- Loss of a proton restores aromaticity.
- Example:C6H6+HNO3 H2SO4→ C6H5NO2+H2O
Other Methods:
- From Diazonium Salts: Reaction of diazonium salts with nitric acid produces nitroaromatics.
- Nucleophilic Substitution: Halonitrobenzenes react to form nitro derivatives under suitable conditions.
Lab Preparation
Principle: In the lab, nitrobenzene is prepared by the nitration of benzene with the mixture of conc. HNO3 and conc. H2SO4 (called nitrating mixture) at 60oC.
NOTE: The purpose of controlling temperature is to prevent further nitration of nitrobenzene is to prevent further nitration of nitrobenzene to m-nitrobenzene.
Refluxing: The process of the heating reaction mixture in a flask fitted with long glass tube so that the vapor or boiling liquid are condensed and returned to the same flask is called refluxing and condenser so fitted vertically is called reflux condenser. Refluxing is done when the reaction mixture requires heating for a long time.
Process: About 50 ml of benzene is placed in RB flask . A well cooled acid mixture of conc. HNO3 and conc.H2SO4 ( 70ml) is added into it, a little by little with shaking and cooling contents of the flask if necessary to keep the temperature below 60oC. After the complex addition of acid mixture, the flask fitted with a reflux condenser (as shown in the figure) and is refluxed (boiled) on the water below 60oC till the yellow oily liquid of nitrobenzene appears on the surface.
Isolation and Separation:
The contents of the RB flask are now transferred into a separating funnel and the nitrobenzene forms upper layer and is separated from the lower acid layer. It is treated with the Na2CO3 solution to remove excess acid and is then washed with water. The lower layer of nitrobenzene is again separated and is dried over anhydrous CaCl2. It is finally distilled using air condenser and collected in fraction in the temperature range of 208oC to 212oC as pure and dry nitrobenzene
Physical Properties of Aromatic Nitro Compounds
- Appearance: Nitro compounds range from colorless to yellow or orange, depending on the number of nitro groups.
- Melting and Boiling Points: Increase with molecular weight and the number of nitro groups.
- Solubility: Insoluble in water but soluble in organic solvents like benzene and alcohol.
Key Note: The aromatic nitro group’s physical properties are influenced by their polar nature and ability to engage in dipole-dipole interactions.
Chemical Properties of Aromatic Nitro Compounds
I) REDUCTION
Nitrobenzene undergoes the reduction in different media to form various products depending on the nature of reducing agent used and the reaction condition .
a) Reduction in acidic medium
Nitrobenzene is reduced to aniline by metal like Zn, Fe, Sn and conc.HCl through the formation of intermediate nitrosobenzene and N-phenyl hydroxylamine.
b) Reduction in natural medium
Nitrobenzene is first reduced to nitrosobenzene and then to N-phenyl hydroxylamine by the neutral reducing agent like Zn and aq.NH4Cl.
c) Reduction in basic medium
Nitrobenzene undergoes the bimolecular reduction in basic medium to form successively azoxybenzene, azobenzene, and hydrazo benzene depending on the nature of the reducing agent used.
d) Electrolytic reduction: Nitrobenzene, on electrolytic reduction in weak acid solution forms aniline and in strong acid solution, it forms N- phenyl hydroxylamine which rearranges to give p-aminophenol.
II ) ELECTROPHILIC SUBSTITUTION REACTION
Electrophile: It is defined as an electron deficient species which accepts an electron pair from an electron rich center of a substrate.The reaction involving substitution of hydrogen of the benzene ring by the initial attack of an electrophile is called electrophilic aromatic substitution reaction which is common in the reaction of aromatic compounds.
In nitrobenzene, benzene ring contains delocalized 6π electrons. So, it is an electron rich system and is thus a good site for the attack of electrophile. Nitrobenzene undergoes electrophilic aromatic substitution in which hydrogen is replaced by various electrophiles.
Electrophilic aromatic substitution reaction in benzene occurs in meta position which is explained by resonance. Nitrobenzene may be represented by following resonating structures.Because of the resonance effect, the nitro group (-NO2) withdraws electrons from the benzene ring and thus decreases electron density more at ortho and para position so that incoming electrophiles attack at electron rich meta position and is thus called meta director.Nitro group ( -NO2), being electron withdrawing group withdraws electrons from the benzene ring and thus deactivates it for further electrophilic aromatic substitution and is thus called ring deactivator.
Nitrobenzene undergoes electrophilic aromatic substitution like halogenation, nitration, sulphonation on metal positions.
I) Halogenation: The substitution of hydrogen of the benzene ring by the halogens is called halogenation.
a) Chlorination: Nitrobenzene undergoes chlorination with chlorine in the presence of Ferric chloride (FeCl3) to m – chloro- nitrobenzene.
b) Bromination: Nitrobenzene undergoes bromination with bromination in the presence of Ferric bromide (FeBr3) to m-Bromo nitrobenzene.
II) Nitration: Nitrobenzene, on nitration with the mixture of conc.HNO3 and conc.H2SO4 form m- dinitrobenzene.
III) Sulphonation: The substitution of hydrogen of benzene ring by sulphonic group ( -SO3H) is called sulphonation. Nitrobenzene undergoes sulphonation with conc. H2SO4 on heating to from m – nitrobenzene sulphonic acid.
Uses and Applications of Aromatic Nitro Compounds
- Industrial Uses:
- Dyes: Nitrobenzene serves as a precursor to aniline, which is essential in dye production.
- Pharmaceuticals: Nitroaromatics are intermediates in drug synthesis.
- Agrochemicals: Used in pesticides and herbicides.
- Explosives:
- Trinitrotoluene (TNT) is widely used in military and mining industries due to its controlled explosive properties.
- Solvents: Nitrobenzene acts as a solvent in organic synthesis and is used in the perfumery industry.
Toxicity and Environmental Impact
Toxic Effects:
- Exposure to aromatic nitro compounds can lead to:
- Hemoglobin oxidation (methemoglobinemia).
- Toxic effects on the liver, kidney, and central nervous system.
- Some nitroaromatics are carcinogenic and mutagenic.
Environmental Concerns:
- Nitroaromatics are persistent in the environment and resist degradation.
- Bioremediation methods, including the use of microorganisms, are being developed to mitigate their impact.