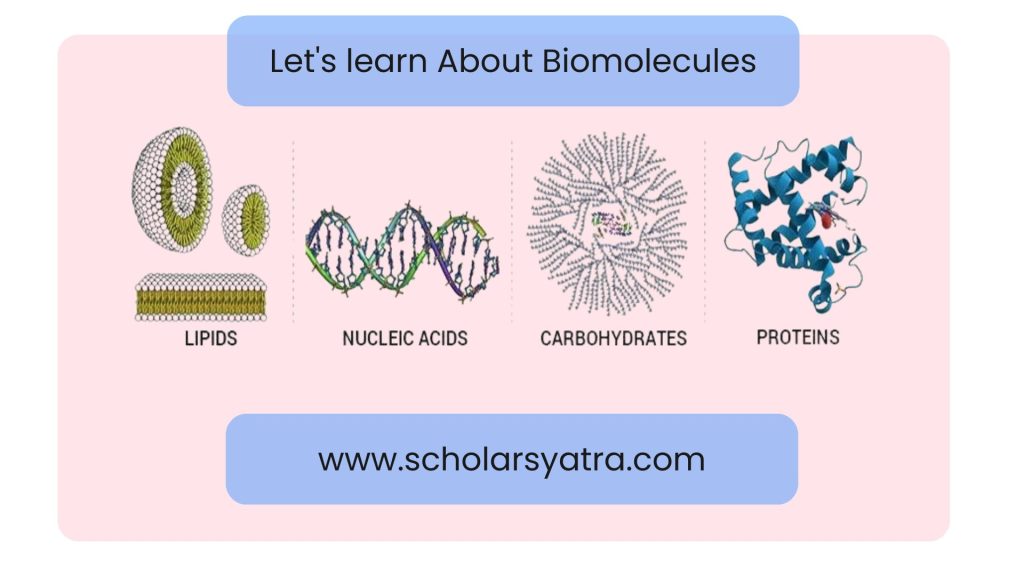
Biomolecules are the essential compounds that sustain life, playing a vital role in the structure, function, and regulation of cells and organisms. These organic molecules, which include carbohydrates, proteins, lipids, and nucleic acids, are fundamental to all biological processes. From providing energy to building cellular structures and storing genetic information, biomolecules are inseparable blocks to the functioning of living organisms. These molecules are crucial for fields like biology, medicine, and biotechnology, as they offer insights into the mechanisms of life itself.
Table of Contents
Toggle
Introduction to Carbohydrates
Carbohydrates are one of the most important complex organic compounds like carbon, hydrogen, and oxygen. Chemically, Carbohydrates are the hydrates of carbon, i.e. carbon having hydroxide. They serve as a primary energy source for living organisms and play structural and storage roles. Chemically, carbohydrates are composed of carbon (C), hydrogen (H), and oxygen (O) atoms, typically with the formula (CH₂O)ₙ, where “n” can vary. Based on their complexity, carbohydrates are classified into three main types: monosaccharides, oligosaccharides, and polysaccharides.
1. Monosaccharides
Monosaccharides are the simplest form of carbohydrates. They are single sugar units, which cannot be hydrolyzed into smaller carbohydrates. These molecules serve as the building blocks for more complex carbohydrates.
Chemical Structure
Monosaccharides follow the general formula (CH₂O)ₙ, where n is typically 3–7. Based on the number of carbon atoms, they can be classified as:
- Trioses (C₃H₆O₃)
- Tetroses (C₄H₈O₄)
- Pentoses (C₅H₁₀O₅)
- Hexoses (C₆H₁₂O₆)
Common Examples:
- Glucose (C₆H₁₂O₆): The most important monosaccharide in human metabolism. Glucose is a primary source of energy for cells.
- Fructose (C₆H₁₂O₆): Found in fruits and honey, it is sweeter than glucose and also serves as an energy source.
- Galactose (C₆H₁₂O₆): Part of lactose, the sugar found in milk.
Functions of Monosaccharides:
- Energy source: Glucose is metabolized in cellular respiration to produce ATP (adenosine triphosphate), which powers biological processes.
- Building blocks: Monosaccharides are used to synthesize oligosaccharides and polysaccharides, which serve structural and storage functions.
2. Oligosaccharides
Oligosaccharides consist of a few (typically 2–10) monosaccharide units linked together by glycosidic bonds which can further cab be split on hydrolysis. They are more complex than monosaccharides but simpler than polysaccharides.
Chemical Structure
Oligosaccharides are formed through the condensation reaction between monosaccharides, creating glycosidic linkages.
Common Examples:
- Sucrose (C₁₂H₂₂O₁₁): A disaccharide composed of glucose and fructose. Commonly known as table sugar.
- Lactose (C₁₂H₂₂O₁₁): A disaccharide found in milk, made up of glucose and galactose.
- Maltose (C₁₂H₂₂O₁₁): A disaccharide formed from two glucose units, commonly found in germinating seeds.
Functions of Oligosaccharides:
- Energy source: Oligosaccharides like sucrose and lactose are broken down into monosaccharides, providing quick energy.
- Prebiotic function: Some oligosaccharides, like raffinose, serve as prebiotics, feeding beneficial gut bacteria.
- Cell recognition: Oligosaccharides attached to proteins or lipids on the cell surface are involved in cell signaling and recognition.
Note: The oligosaccharides are further classified into the following types:
- Disaccharides: E.g. Sucrose, Maltose
- Trisaccharides: E.g. Rafinose, Maltotriose
- Tetrasaccharides: E.g Stachyose
3. Polysaccharides
Polysaccharides are long chains of monosaccharide units (often hundreds or thousands) linked by glycosidic bonds. They are complex carbohydrates, usually serving as energy storage or structural materials. They are insoluble in water and do not taste sweet in taste.
Chemical Structure
Polysaccharides can be linear or branched, and the glycosidic bonds can vary, leading to different properties.
Common Examples:
- Starch (C₆H₁₀O₅)ₙ: A storage polysaccharide in plants, consisting of amylose (linear) and amylopectin (branched). It is made up of glucose units.
- Glycogen (C₆H₁₀O₅)ₙ: The animal equivalent of starch, stored in the liver and muscles. It is highly branched, allowing rapid glucose release.
- Cellulose (C₆H₁₀O₅)ₙ: A structural polysaccharide found in the cell walls of plants. It is composed of linear chains of glucose and provides rigidity and strength to plant cells.
- Chitin (C₈H₁₃O₅N)ₙ: A structural polysaccharide found in the exoskeletons of arthropods and the cell walls of fungi.
Functions of Polysaccharides:
- Energy storage: Starch (in plants) and glycogen (in animals) store energy that can be mobilized when needed.
- Structural support: Cellulose in plants provides structural integrity, and chitin in animals and fungi offers protection and support.
- Water retention: Certain polysaccharides, like mucopolysaccharides, help retain water, providing lubrication in biological systems such as joints and connective tissue.
Functions of Carbohydrates
Carbohydrates play several essential roles in biological systems:
- Energy production: Carbohydrates, particularly glucose, are the main energy source for cellular processes.
- Storage of energy: Polysaccharides like starch and glycogen store energy that can be released during metabolism.
- Structural roles: Carbohydrates like cellulose and chitin provide structural support in plants, fungi, and arthropods.
- Cell recognition and signaling: Oligosaccharides attached to proteins and lipids on cell surfaces are critical for cell communication, recognition, and immune response.
Carbohydrates are crucial biomolecules, providing energy, storage, and structural support across a wide range of organisms. By understanding the three types—monosaccharides, oligosaccharides, and polysaccharides—and their specific functions, we can appreciate the central role they play in life’s biological processes.
Introduction to Proteins
Proteins are one of the most versatile and essential biomolecules in living organisms. They perform a wide array of functions, including structural support, catalyzing biochemical reactions, transporting molecules, and more. Proteins are polymers of amino acids linked together by peptide bonds, and their structure determines their function. In this topic, we will explore the classification of proteins based on their structure, chemical composition, and arrangement of polypeptide chains, followed by an overview of their key biological functions.
1. Protein Structure: Hierarchical Levels
Proteins have a complex structure, which can be described at four hierarchical levels: primary, secondary, tertiary, and quaternary structures.
a. Primary Structure
- Definition: The primary structure of a protein refers to the linear sequence of amino acids in the polypeptide chain. This sequence is determined by the genetic code.
- Example: A simple polypeptide with a sequence such as Glycine-Alanine-Valine-Phenylalanine represents its primary structure.
b. Secondary Structure
- Definition: The secondary structure refers to the local folding of the polypeptide chain into regular patterns, stabilized by hydrogen bonds between the peptide backbone.
- Types:
- α-helix: A right-handed coiled structure where the carbonyl oxygen of one amino acid forms a hydrogen bond with the amide hydrogen of an amino acid four residues away.
- β-pleated sheet: A structure where segments of the polypeptide chain lie side by side, forming hydrogen bonds between backbone atoms.
- Example: Keratin (α-helix) and silk fibroin (β-pleated sheet).
c. Tertiary Structure
- Definition: The tertiary structure is the overall three-dimensional shape of a single polypeptide chain, resulting from interactions between the side chains (R-groups) of amino acids.
- Forces Involved:
- Hydrophobic interactions
- Hydrogen bonds
- Disulfide bridges (between cysteine residues)
- Ionic bonds (between charged side chains)
- Example: Myoglobin, a globular protein with a complex 3D structure that binds oxygen in muscle tissue.
d. Quaternary Structure
- Definition: The quaternary structure involves the assembly of multiple polypeptide chains (subunits) into a single functional protein complex. This level is only present in multimeric proteins.
- Example: Hemoglobin, which consists of four polypeptide chains (two alpha and two beta subunits), is an example of a protein with a quaternary structure.
2. Classification of Proteins
Proteins can be classified based on three criteria: their chemical composition, structure, and the arrangement of their polypeptide chains.
A. Classification Based on Chemical Composition
Proteins can be classified as simple or conjugated based on the presence of non-protein components.
- Simple Proteins: These proteins consist only of amino acids. When hydrolyzed, they yield only amino acids.
- Examples: Albumins (found in egg whites), and globulins (found in blood plasma).
- Conjugated Proteins: These proteins contain a non-protein component known as a prosthetic group, which may be a metal ion, lipid, carbohydrate, or nucleic acid.
- Examples:
- Glycoproteins: Proteins with a carbohydrate prosthetic group, e.g., antibodies.
- Lipoproteins: Proteins with lipid groups, e.g., plasma lipoproteins involved in cholesterol transport.
- Hemoproteins: Proteins with a heme group, e.g., hemoglobin.
- Examples:
B. Classification Based on Structure
Proteins can also be classified into three main categories based on their overall structure.
- Fibrous Proteins:
- Structure: Long, rod-like, or filamentous proteins. They are usually insoluble in water and serve structural roles.
- Examples:
- Collagen: Found in connective tissues like tendons and ligaments.
- Keratin: Found in hair, nails, and the outer layer of skin.
- Functions: Provide structural support, rigidity, and strength to cells and tissues.
- Globular Proteins:
- Structure: These proteins have a spherical or compact shape and are usually soluble in water.
- Examples:
- Enzymes: Such as amylase and trypsin, which catalyze biochemical reactions.
- Hemoglobin: Transports oxygen in the blood.
- Functions: Serve as enzymes, transporters, regulators, and antibodies.
- Intermediate (Mixed) Proteins:
- These proteins exhibit characteristics of both fibrous and globular proteins. They have a somewhat elongated shape but also have functional flexibility.
- Example: Fibrinogen, which is involved in blood clotting.
C. Classification Based on the Arrangement of Polypeptide Chains
Proteins can also be classified based on how their polypeptide chains are arranged or organized:
- Monomeric/Primary Proteins: Contain a single polypeptide chain.
- Example: Myoglobin.
- Multimeric Proteins: Contain more than one polypeptide chain, which may be identical or different.
- Homomultimeric Proteins: Made up of identical polypeptide subunits.
- Example: Lactate dehydrogenase.
- Heteromultimeric Proteins: Contain different polypeptide subunits.
- Example: Hemoglobin, which contains two types of subunits (alpha and beta).
- Homomultimeric Proteins: Made up of identical polypeptide subunits.
3. Functions of Proteins
Proteins have a vast array of biological functions, some of which are essential for the maintenance and regulation of life processes. Below are the key functions of proteins:
- Structural Support:
- Proteins such as collagen, keratin, and elastin provide structural integrity and support to cells and tissues.
- Catalysis (Enzymatic Activity):
- Enzymes are proteins that catalyze biochemical reactions, speeding them up without being consumed. Examples include amylase (digestion of starch) and DNA polymerase (replication of DNA).
- Transport:
- Proteins such as hemoglobin transport oxygen, while membrane proteins like ion channels and pumps regulate the movement of molecules across cell membranes.
- Defense (Immune Response):
- Antibodies (immunoglobulins) are proteins that recognize and neutralize foreign invaders such as bacteria and viruses.
- Regulation (Hormones):
- Some hormones are proteins, like insulin, which regulates blood glucose levels. Others, like growth hormone, regulate cell growth and development.
- Movement:
- Motor proteins such as actin and myosin are involved in muscle contraction and other forms of cellular movement.
- Storage:
- Some proteins, like ferritin, store important nutrients. Ferritin stores iron in the liver.
- Cell Communication:
- Receptor proteins in the cell membrane bind to signaling molecules (like hormones) and initiate a response inside the cell.
Proteins are extraordinary biomolecules, vital for nearly every biological process. Their classification based on structure, chemical composition, and arrangement of polypeptide chains highlights their diversity in form and function.
Introduction to Lipids
Lipids are a diverse group of hydrophobic or amphipathic molecules that play crucial roles in living organisms. Unlike carbohydrates and proteins, lipids do not have a specific monomer unit but are characterized by their insolubility in water and solubility in non-polar solvents. Lipids serve as energy storage molecules, structural components of cell membranes, and signaling molecules. Here, we will cover the classification of lipids based on their structure and chemical composition, and discuss their key functions in biological systems.
1. Classification of Lipids
Lipids can be classified based on their chemical composition and structure into three main categories: simple lipids, compound lipids, and derived lipids.
A. Simple Lipids
Simple lipids are esters of fatty acids with alcohols. They are the basic forms of lipids and include fats, oils, and waxes.
- Fats and Oils (Triglycerides or Triacylglycerols)
- Structure: Triglycerides are composed of three fatty acid molecules esterified to a glycerol molecule. Fatty acids can be either saturated (no double bonds) or unsaturated (one or more double bonds).
- Saturated fats: Have no double bonds between carbon atoms (e.g., palmitic acid, stearic acid).
- Unsaturated fats: Have one or more double bonds (e.g., oleic acid, linoleic acid).
- Examples:
- Fats: Solid at room temperature, such as butter and lard.
- Oils: Liquid at room temperature, such as olive oil and sunflower oil.
- Functions:
- Energy storage (long-term): Triglycerides store energy efficiently in adipose tissue.
- Insulation and protection: Fat provides insulation against heat loss and mechanical cushioning for organs.
- Structure: Triglycerides are composed of three fatty acid molecules esterified to a glycerol molecule. Fatty acids can be either saturated (no double bonds) or unsaturated (one or more double bonds).
- Waxes
- Structure: Waxes are esters of long-chain fatty acids and long-chain alcohols.
- Examples: Beeswax, carnauba wax, and lanolin.
- Functions: Provide protective coatings in plants (cuticles of leaves) and animals (skin, feathers, fur), and prevent water loss.
B. Compound (Complex) Lipids
Compound lipids are esters of fatty acids with additional functional groups such as phosphate, nitrogenous bases, or carbohydrates. These lipids are involved in structural and functional roles within the cell.
- Phospholipids
- Structure: Phospholipids consist of two fatty acids, a glycerol backbone, and a phosphate group attached to a polar head group (like choline or ethanolamine).
- Examples:
- Phosphatidylcholine (Lecithin): Found in cell membranes.
- Phosphatidylserine: Important in signaling processes.
- Functions:
- Structural role: Phospholipids are key components of the lipid bilayer in cell membranes, providing a barrier and structural support.
- Membrane fluidity: Their amphipathic nature allows them to form bilayers, with hydrophilic heads facing outward and hydrophobic tails inward.
- Cell signaling: Phospholipids participate in signaling pathways, especially phosphoinositides in intracellular signaling.
- Glycolipids
- Structure: Glycolipids consist of a carbohydrate group attached to a lipid molecule (usually a fatty acid and sphingosine).
- Examples:
- Cerebrosides: Contain a single sugar (glucose or galactose).
- Gangliosides: Contain multiple sugar units and are found in the nervous system.
- Functions:
- Cell recognition: Glycolipids are found on the outer surface of the plasma membrane and play a role in cell-cell recognition.
- Immune response: Glycolipids are involved in immune system processes and blood group antigens.
- Lipoproteins
- Structure: Lipoproteins consist of lipids bound to proteins. They facilitate the transport of lipids in the bloodstream.
- Examples:
- Low-Density Lipoprotein (LDL): Transports cholesterol to tissues.
- High-Density Lipoprotein (HDL): Transports cholesterol away from tissues to the liver for excretion.
- Functions:
- Transport: Lipoproteins carry lipids like cholesterol and triglycerides through the bloodstream, regulating lipid metabolism.
C. Derived Lipids
Derived lipids are substances derived from simple or compound lipids through hydrolysis. These include fatty acids, glycerol, steroids, and other lipid-like molecules.
- Fatty Acids
- Structure: Long hydrocarbon chains with a carboxyl group (-COOH) at one end.
- Examples:
- Saturated fatty acids: Palmitic acid (C16H32O2), Stearic acid (C18H36O2).
- Unsaturated fatty acids: Oleic acid (C18H34O2), Linoleic acid (C18H32O2).
- Functions:
- Serve as building blocks for triglycerides and phospholipids.
- Play a role in energy storage and metabolic processes.
- Steroids
- Structure: Steroids have a four-ring structure made of carbon atoms.
- Examples:
- Cholesterol: Precursor to steroid hormones and an essential component of cell membranes.
- Steroid hormones: Includes hormones like cortisol, testosterone, and estrogen.
- Functions:
- Cholesterol stabilizes cell membranes and serves as a precursor to steroid hormones.
- Steroid hormones regulate various physiological processes such as metabolism, immune response, and reproduction.
- Terpenes
- Structure: Terpenes are derived from isoprene units (C5H8).
- Examples: Vitamin A, carotenoids, and essential oils.
- Functions: Terpenes are involved in biological processes such as photosynthesis (carotenoids) and vision (vitamin A).
2. Functions of Lipids
Lipids perform a variety of vital functions in the body, from energy storage to serving as key structural and signaling molecules.
- Energy Storage:
- Lipids store energy more efficiently than carbohydrates. One gram of fat provides about 9 kcal of energy, compared to 4 kcal per gram of carbohydrates.
- Triglycerides stored in adipose tissue serve as long-term energy reserves for the body.
- Cell Membrane Structure:
- Phospholipids and cholesterol are major components of cell membranes. Phospholipids form the bilayer that provides structural integrity and regulates the passage of substances in and out of cells.
- Cholesterol helps maintain membrane fluidity and stability, especially in changing temperatures.
- Insulation and Protection:
- In animals, fats provide insulation, reducing heat loss and protecting internal organs from mechanical shock.
- Signaling Molecules:
- Lipids are involved in cellular signaling pathways. Steroid hormones (like cortisol and estrogen) regulate metabolism, reproduction, and immune response.
- Eicosanoids, derived from fatty acids like arachidonic acid, play a role in inflammation and other signaling processes.
- Transport of Nutrients:
- Lipids are essential for the absorption and transport of fat-soluble vitamins (A, D, E, and K). Lipoproteins in the bloodstream transport cholesterol, triglycerides, and other lipids to tissues.
- Waterproofing:
- Waxes act as protective barriers to prevent water loss in plants and animals. For example, the cuticle of plant leaves and the waxy coating on bird feathers prevent water absorption and dehydration.
Introduction to Amino Acids
Amino acids are the building blocks of proteins, which are critical to almost every biological process. They contain an amino group (-NH₂), a carboxyl group (-COOH), a hydrogen atom, and a unique side chain (R-group), all attached to a central carbon atom (α-carbon). Amino acids join together through peptide bonds to form polypeptide chains, which fold into functional proteins. There are 20 standard amino acids, each differing in the structure of their R-group, and they are categorized based on their chemical properties and biological functions.
However, pentose, nitrogenous bases, and phosphoric acids refer to nucleotides. Nucleotides form the building blocks of nucleic acids (DNA and RNA). They consist of a nitrogenous base, a pentose sugar (ribose or deoxyribose), and a phosphate group. Let’s clarify both concepts. We will first cover amino acids, then nucleotides.
Part I: Amino Acids – Structure and Classification
Amino acids can be classified based on the nature of their side chains (R-groups), their polarity, or their nutritional importance. Here, we’ll explore the amino acids based on the chemical nature of their side chains, their structures, and their functions in the body.
1. Structure of Amino Acids
All amino acids share a basic structure but differ in the composition of their R-group (side chain), which determines their chemical properties.
- Basic Structure: H2N−CHR−COOHH_2N−CHR−COOHH2N−CHR−COOH Where:
- H₂N: Amino group
- COOH: Carboxyl group
- R: Variable side chain (specific to each amino acid)
- C: Central carbon atom (α-carbon)
- H: Hydrogen atom attached to the α-carbon
2. Amino Acids Based on Side Chain Properties
Amino acids can be classified into three broad categories based on the properties of their R-groups:
A. Non-Polar (Hydrophobic) Amino Acids
- These amino acids have hydrophobic side chains that do not interact favorably with water. They tend to be buried in the interior of protein structures.
- Examples:
- Glycine (Gly, G): The simplest amino acid with just a hydrogen atom as its R-group.
- Alanine (Ala, A): Has a methyl group (-CH₃) as its side chain.
- Valine (Val, V), Leucine (Leu, L), Isoleucine (Ile, I): Have branched side chains.
- Proline (Pro, P): A cyclic amino acid that introduces kinks in polypeptide chains.
- Phenylalanine (Phe, F): Contains a benzyl group, adding bulkiness to the side chain.
- Functions:
- Hydrophobic amino acids stabilize protein structures by forming the hydrophobic core.
- Phenylalanine is a precursor for neurotransmitters like dopamine.
B. Polar, Uncharged Amino Acids
- These amino acids have side chains that can form hydrogen bonds with water but do not carry a net charge at physiological pH.
- Examples:
- Serine (Ser, S) and Threonine (Thr, T): Contain hydroxyl (-OH) groups.
- Asparagine (Asn, N) and Glutamine (Gln, Q): Have amide groups (-CONH₂).
- Cysteine (Cys, C): Contains a thiol (-SH) group, which can form disulfide bonds.
- Functions:
- Serine and threonine participate in enzyme catalysis and are involved in phosphorylation reactions.
- Cysteine plays a critical role in protein folding through disulfide bond formation, which stabilizes protein structure.
C. Charged Amino Acids (Acidic and Basic)
- These amino acids have ionizable side chains that can carry a positive or negative charge at physiological pH.
- Acidic Amino Acids (Negatively Charged):
- Aspartic acid (Asp, D) and Glutamic acid (Glu, E): Their side chains contain carboxyl groups (-COOH) that lose protons at physiological pH, giving them a negative charge.
- Basic Amino Acids (Positively Charged):
- Lysine (Lys, K): Has an amino group (-NH₂) that is protonated at physiological pH.
- Arginine (Arg, R): Contains a highly basic guanidino group.
- Histidine (His, H): Has an imidazole group, which can act as both an acid and a base in enzymatic reactions.
- Acidic Amino Acids (Negatively Charged):
- Functions:
- Charged amino acids are involved in maintaining protein solubility and are critical for the catalytic activity of enzymes.
- Basic amino acids are found in histones, proteins that bind to DNA, which is negatively charged.
3. Special Function Amino Acids
- Methionine (Met, M): Contains a sulfur atom and is the first amino acid in protein synthesis.
- Tyrosine (Tyr, Y): Precursor for hormones like thyroxine and neurotransmitters like dopamine.
- Tryptophan (Trp, W): Precursor for serotonin, a neurotransmitter involved in mood regulation.
Part II: Nucleotides – Structure and Types
Nucleotides, are (pentose sugars, nitrogenous bases, and phosphoric acids), are the building blocks of nucleic acids (DNA and RNA). Each nucleotide consists of three components:
- Pentose Sugar: Either ribose (in RNA) or deoxyribose (in DNA).
- Nitrogenous Base: Can be a purine or pyrimidine.
- Phosphate Group: Attaches to the 5′ carbon of the sugar, forming the backbone of nucleic acids.
1. Structure of Nucleotides
Nucleotides are composed of three major components:
- Pentose Sugar: Ribose in RNA and deoxyribose in DNA.
- Nitrogenous Base: Attached to the 1′ carbon of the pentose sugar. There are two types of nitrogenous bases:
- Purines: Adenine (A) and Guanine (G).
- Pyrimidines: Cytosine (C), Thymine (T, in DNA), and Uracil (U, in RNA).
- Phosphate Group: Attached to the 5′ carbon of the pentose sugar. Multiple phosphates may be attached (e.g., ATP has three phosphate groups).
2. Types of Nucleotides
Based on their nitrogenous bases, nucleotides are divided into two categories:
A. Purines
- Structure: Have a double-ring structure.
- Examples:
- Adenine (A)
- Guanine (G)
- Functions:
- Purines are critical components of both DNA and RNA.
- Adenine forms ATP (adenosine triphosphate), which is the energy currency of the cell.
B. Pyrimidines
- Structure: Have a single-ring structure.
- Examples:
- Cytosine (C), Thymine (T) (in DNA), and Uracil (U) (in RNA).
- Functions:
- Pyrimidines pair with purines in the double helix of DNA (A pairs with T, G pairs with C).
- Uracil replaces thymine in RNA.
3. Functions of Nucleotides
- Genetic Information: Nucleotides form the backbone of DNA and RNA, which carry genetic information in cells.
- Energy Transfer: ATP is a nucleotide that stores and transfers energy in cells.
- Cellular Signaling: cAMP and cGMP are nucleotide derivatives involved in signal transduction pathways.
Types of Nucleic Acids
Introduction to Nucleic Acids (DNA and RNA)
Nucleic acids—DNA (Deoxyribonucleic Acid) and RNA (Ribonucleic Acid)—are the fundamental molecules that store and transfer genetic information in cells. DNA holds the genetic blueprint of an organism, while RNA plays several roles in expressing that information, especially during protein synthesis. Both DNA and RNA are composed of nucleotides, which are the building blocks of these molecules.
1. Structure of DNA (Deoxyribonucleic Acid)
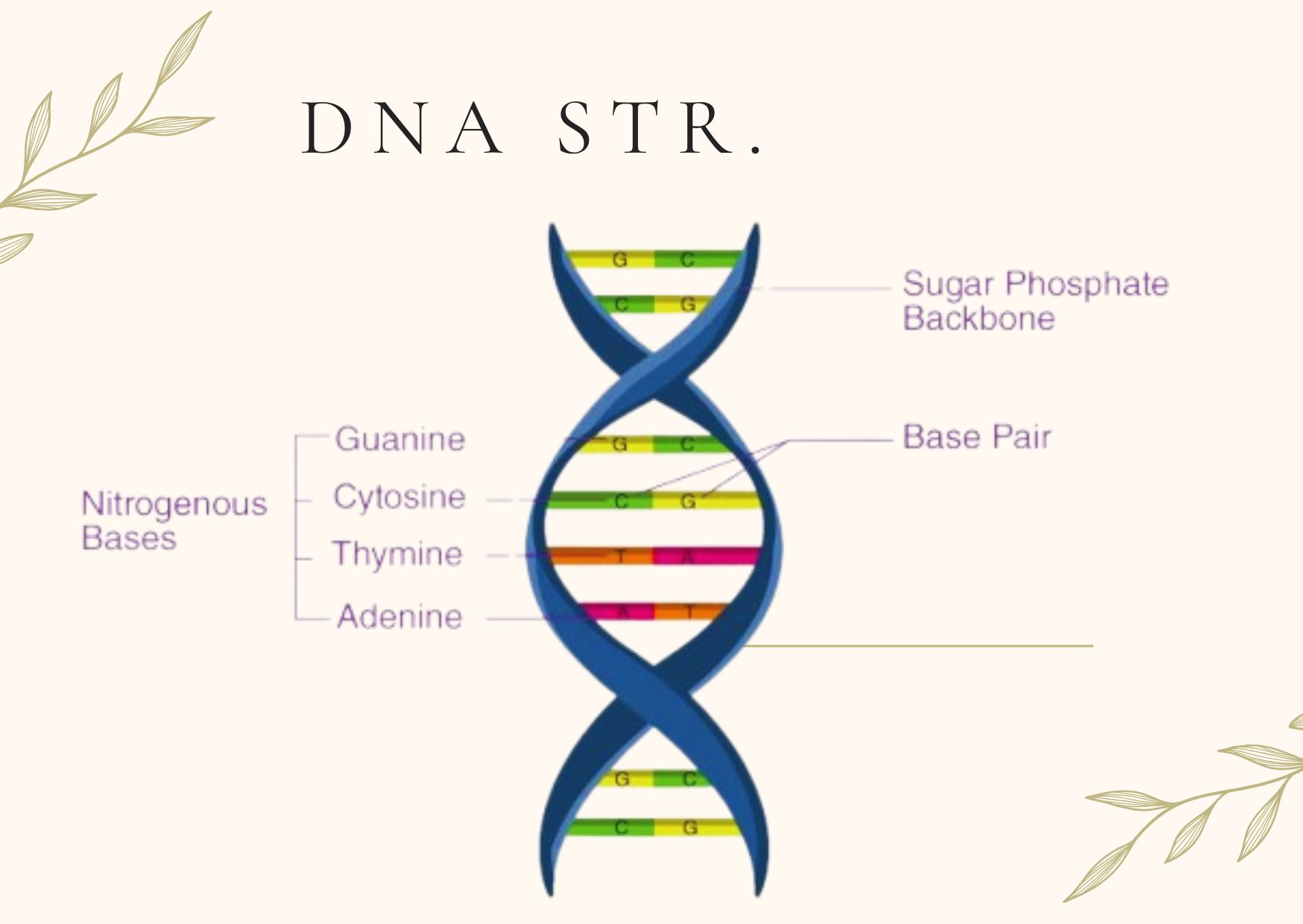
A. Components of DNA
DNA is a long, double-stranded molecule composed of repeating units called nucleotides. Each nucleotide consists of three parts:
- Pentose Sugar (Deoxyribose): A five-carbon sugar that lacks an oxygen atom at the 2′ position compared to ribose in RNA.
- Nitrogenous Base: DNA contains four nitrogenous bases, divided into two categories:
- Purines: Adenine (A) and Guanine (G)
- Pyrimidines: Cytosine (C) and Thymine (T)
- Phosphate Group: Links the 5′ carbon of one sugar to the 3′ carbon of the next, forming the sugar-phosphate backbone.
B. Double-Helix Structure of DNA
- Watson-Crick Model: DNA exists as a double helix, where two complementary strands are wound around each other. The two strands run in opposite directions (antiparallel), with one strand running 5′ to 3′ and the other 3′ to 5′.
- Base Pairing: Nitrogenous bases on opposite strands pair through hydrogen bonds:
- Adenine (A) pairs with Thymine (T) through two hydrogen bonds.
- Guanine (G) pairs with Cytosine (C) through three hydrogen bonds.
- Major and Minor Grooves: The helical structure of DNA creates grooves that allow access to the nitrogenous bases by proteins such as transcription factors.
C. DNA Replication
- Semi-Conservative Replication: Each strand of the DNA molecule serves as a template for the synthesis of a new complementary strand. The result is two DNA molecules, each composed of one old strand and one newly synthesized strand.
- Key Enzymes:
- Helicase: Unwinds the double helix.
- DNA Polymerase: Adds new nucleotides to the growing strand in the 5′ to 3′ direction.
- Ligase: Seals nicks in the sugar-phosphate backbone, particularly on the lagging strand where Okazaki fragments are formed.
2. Structure of RNA (Ribonucleic Acid)
A. Components of RNA
Like DNA, RNA is also made up of nucleotides, but with a few key differences:
- Pentose Sugar (Ribose): Unlike deoxyribose, ribose contains a hydroxyl group (-OH) at the 2′ position, making RNA more reactive and less stable than DNA.
- Nitrogenous Base: RNA contains Uracil (U) instead of Thymine (T):
- Purines: Adenine (A) and Guanine (G)
- Pyrimidines: Cytosine (C) and Uracil (U)
- Phosphate Group: Same as in DNA, linking the nucleotides together to form a backbone.
B. Single-Stranded Nature of RNA
- Single-Stranded Molecule: RNA is generally single-stranded, but it can form complex secondary structures through intramolecular base pairing (e.g., hairpins and loops).
- Base Pairing: In RNA, Adenine (A) pairs with Uracil (U), while Guanine (G) pairs with Cytosine (C).
3. Types of RNA
There are several types of RNA, each with a specific role in the process of gene expression and protein synthesis:
A. Messenger RNA (mRNA)
- Structure: Single-stranded RNA that carries genetic information from DNA in the nucleus to the ribosome, where proteins are synthesized.
- Function: Acts as a template for the sequence of amino acids in proteins. Each set of three nucleotides (codon) in mRNA corresponds to a specific amino acid.
B. Transfer RNA (tRNA)
- Structure: tRNA is a small RNA molecule (~75-95 nucleotides) that folds into a cloverleaf structure due to intramolecular base pairing. It contains an anticodon region that recognizes the complementary codon on mRNA.
- Function: tRNA delivers the appropriate amino acid to the ribosome during protein synthesis, matching the anticodon to the mRNA codon to ensure the correct amino acid is added to the growing polypeptide chain.
C. Ribosomal RNA (rRNA)
- Structure: rRNA molecules, together with proteins, make up the ribosome. rRNA has complex secondary and tertiary structures and is highly conserved across species.
- Function: rRNA forms the core of the ribosome’s structure and catalyzes the formation of peptide bonds between amino acids during translation (protein synthesis).
D. Other Types of RNA
- Small Nuclear RNA (snRNA): Involved in the splicing of pre-mRNA in the nucleus, forming part of the spliceosome.
- MicroRNA (miRNA) and Small Interfering RNA (siRNA): Play roles in regulating gene expression by degrading mRNA or blocking its translation.
4. Functions of DNA and RNA
A. Functions of DNA
- Genetic Information Storage: DNA stores the genetic instructions required for the development, function, and reproduction of all living organisms and some viruses.
- Replication: DNA replicates itself before cell division, ensuring that genetic information is passed on to daughter cells.
- Gene Expression: While DNA holds the instructions for proteins, it cannot leave the nucleus (in eukaryotes). Instead, it is transcribed into RNA, which carries out the instructions.
B. Functions of RNA
- Protein Synthesis (Translation): RNA is directly involved in the synthesis of proteins:
- mRNA: Carries the genetic code from DNA to the ribosome.
- tRNA: Delivers amino acids to the ribosome to be incorporated into the growing polypeptide chain.
- rRNA: Helps form the structure of ribosomes and catalyzes peptide bond formation.
- Regulation of Gene Expression: Certain RNAs (like miRNA and siRNA) play a role in regulating gene expression post-transcriptionally by degrading mRNA or inhibiting translation.
- RNA as Catalysts (Ribozymes): Some RNA molecules, such as rRNA in the ribosome, have catalytic activity, performing enzymatic functions.
5. Differences Between DNA and RNA
| Feature | DNA (Deoxyribonucleic Acid) | RNA (Ribonucleic Acid) |
|---|---|---|
| Sugar | Deoxyribose | Ribose |
| Strands | Double-stranded (helical) | Single-stranded |
| Nitrogenous Bases | Adenine, Thymine, Guanine, Cytosine | Adenine, Uracil, Guanine, Cytosine |
| Location | Nucleus (in eukaryotes) | Nucleus and cytoplasm |
| Function | Stores genetic information | Transfers genetic information for protein synthesis, gene regulation |
6. DNA and RNA in Protein Synthesis
The central dogma of molecular biology describes the flow of genetic information from DNA to RNA to proteins:
- Transcription (DNA to RNA):
- DNA is transcribed into mRNA in the nucleus.
- Enzyme: RNA polymerase binds to the promoter region of a gene, and synthesizes mRNA by matching complementary RNA nucleotides to the DNA template.
- Translation (RNA to Protein):
- The mRNA is translated into a protein at the ribosome in the cytoplasm.
- tRNA: Brings amino acids to the ribosome, where they are assembled into polypeptides based on the sequence of codons in the mRNA.
Introduction to Enzymes
Enzymes are biological catalysts that speed up chemical reactions in living organisms without being consumed in the process. They are essential for various metabolic processes, including digestion, energy production, and DNA replication. Enzymes are typically proteins, although some RNA molecules (ribozymes) can also function as enzymes.
Characteristics of Enzymes:
- Specificity: Enzymes are highly specific to their substrates (the molecules they act upon). The specificity is determined by the enzyme’s active site, a unique pocket that fits only particular substrates.
- Catalytic Efficiency: Enzymes lower the activation energy required for a reaction to proceed, thereby accelerating the reaction rate. This allows biochemical processes to occur efficiently under the mild conditions of temperature and pH found in cells.
- Regulation: Enzyme activity can be regulated by factors like temperature, pH, and the presence of inhibitors or activators. Cells also regulate enzyme production and degradation based on metabolic needs.
- Reusability: Enzymes are not consumed in the reaction. Once they catalyze a reaction, they are free to bind to new substrate molecules and repeat the process.
Enzyme Classification:
Enzymes are classified based on the type of reaction they catalyze:
- Oxidoreductases: Involved in oxidation-reduction reactions (e.g., dehydrogenases, oxidases).
- Transferases: Transfer functional groups from one molecule to another (e.g., kinases, transaminases).
- Hydrolases: Catalyze the breaking of bonds by adding water (e.g., proteases, lipases).
- Lyases: Remove groups from substrates without hydrolysis or oxidation (e.g., decarboxylases).
- Isomerases: Rearrange atoms within a molecule (e.g., isomerases).
- Ligases: Catalyze the joining of two molecules with the input of energy (e.g., DNA ligase).
Examples of Enzymes:
- Amylase: Breaks down starch into sugars during digestion.
- DNA Polymerase: Synthesizes new DNA strands during DNA replication.
- Lipase: Breaks down fats into fatty acids and glycerol.
- Lactase: Breaks down lactose (milk sugar) into glucose and galactose.
Functions of Enzymes:
- Metabolism: Enzymes drive metabolic pathways that sustain life, including the breakdown of nutrients and the synthesis of cellular components.
- Cell Signaling and Regulation: Enzymes regulate processes like cell signaling and gene expression.
- Industrial Applications: Enzymes are used in biotechnology, pharmaceuticals, food processing (e.g., brewing, dairy), and more.
Happy Learning Guys!