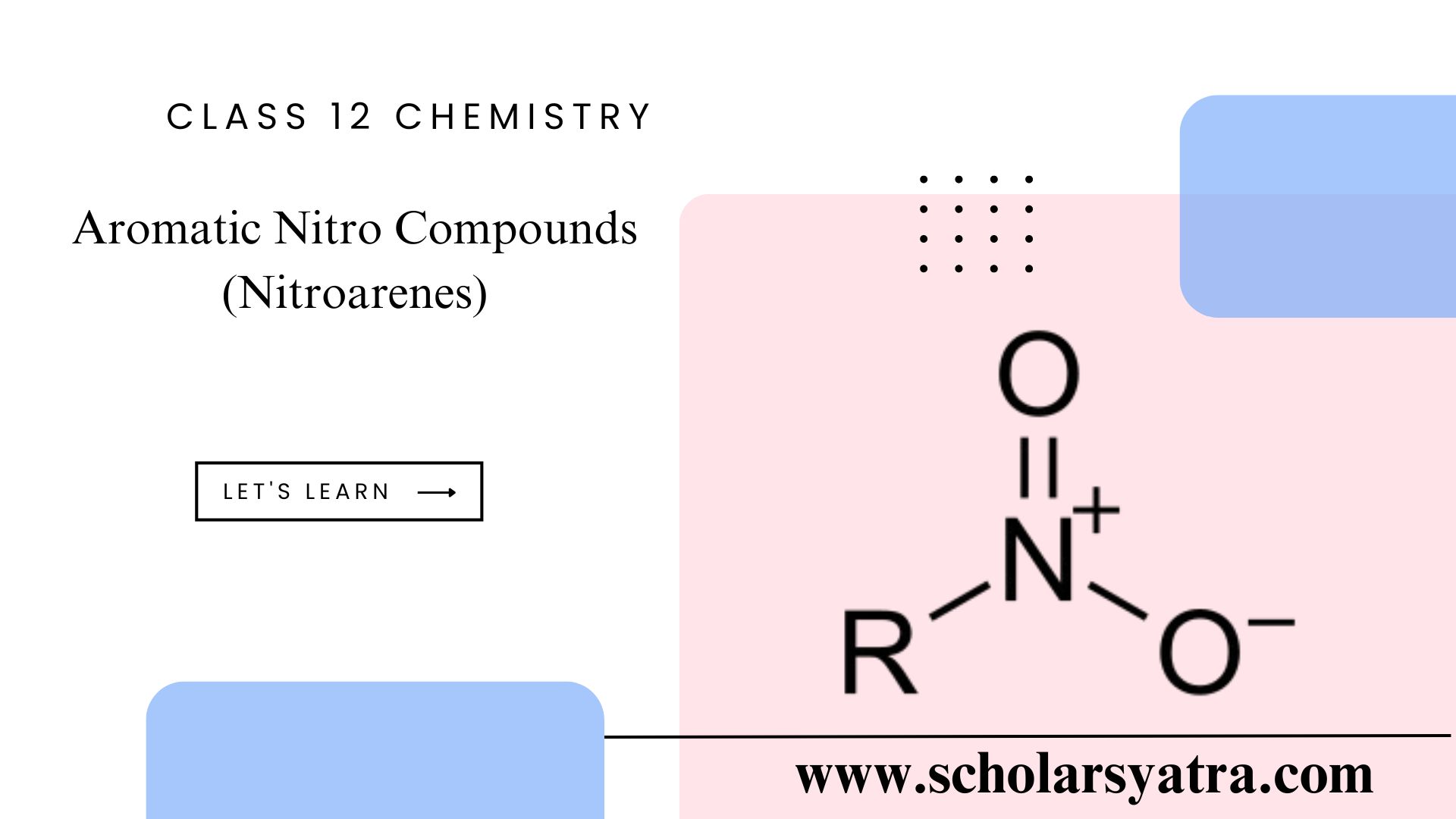
Aromatic Nitro Compounds
Compounds in which the nitro group ( -NO2) is directly bonded to the benzene (aromatic) ring are called aromatic nitro compounds or simply nitroarenes. These

Compounds in which the nitro group ( -NO2) is directly bonded to the benzene (aromatic) ring are called aromatic nitro compounds or simply nitroarenes. These
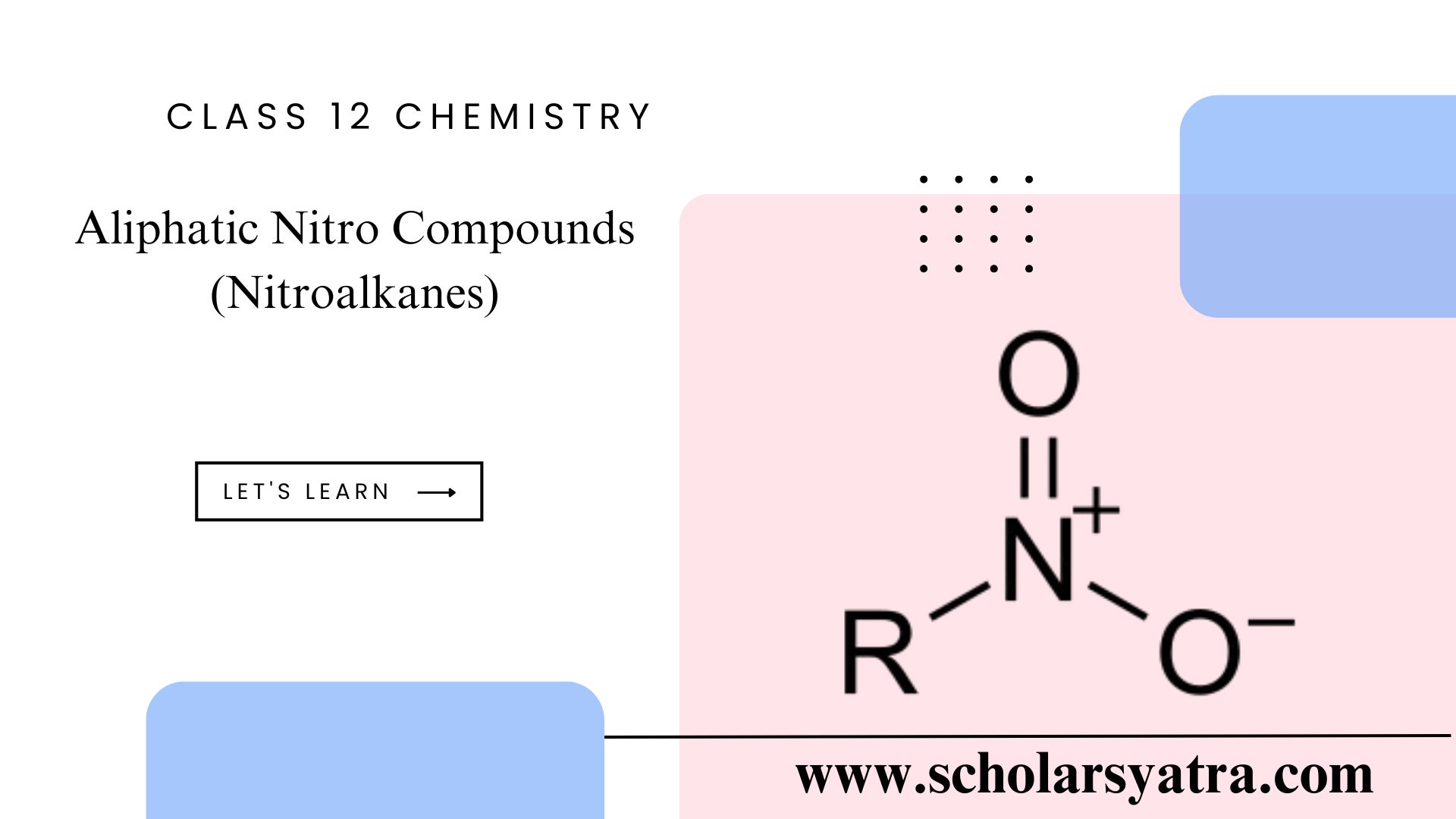
Aliphatic nitro compounds are organic compounds that contain one or more nitro groups (-NO₂) attached to an aliphatic carbon atom. These compounds are known for
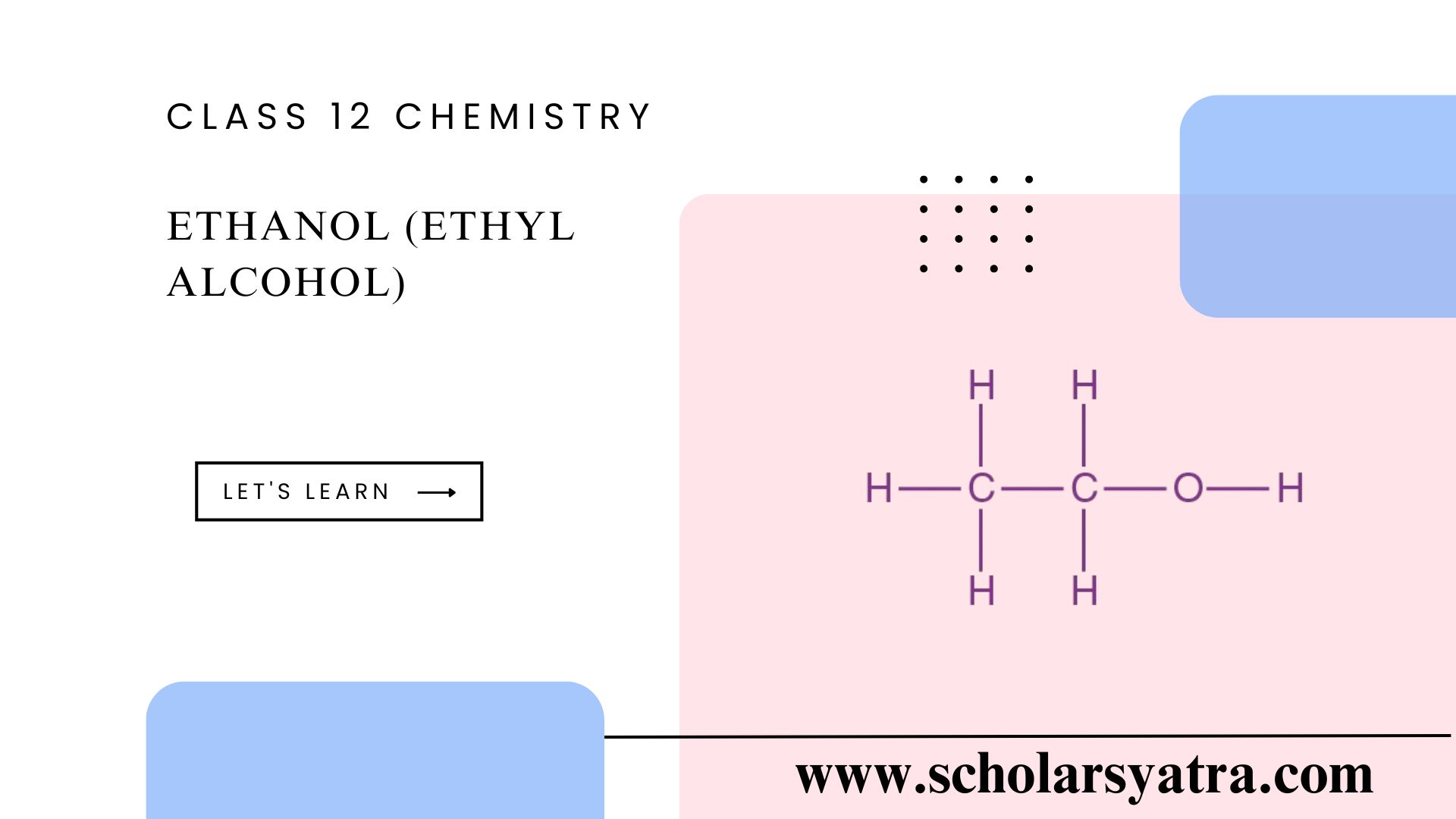
Ethanol, or ethyl alcohol, is a simple aliphatic alcohol with the chemical formula C₂H₅OH. Ethanol (Ethyl Alcohol) is a colorless, volatile, and flammable liquid with a
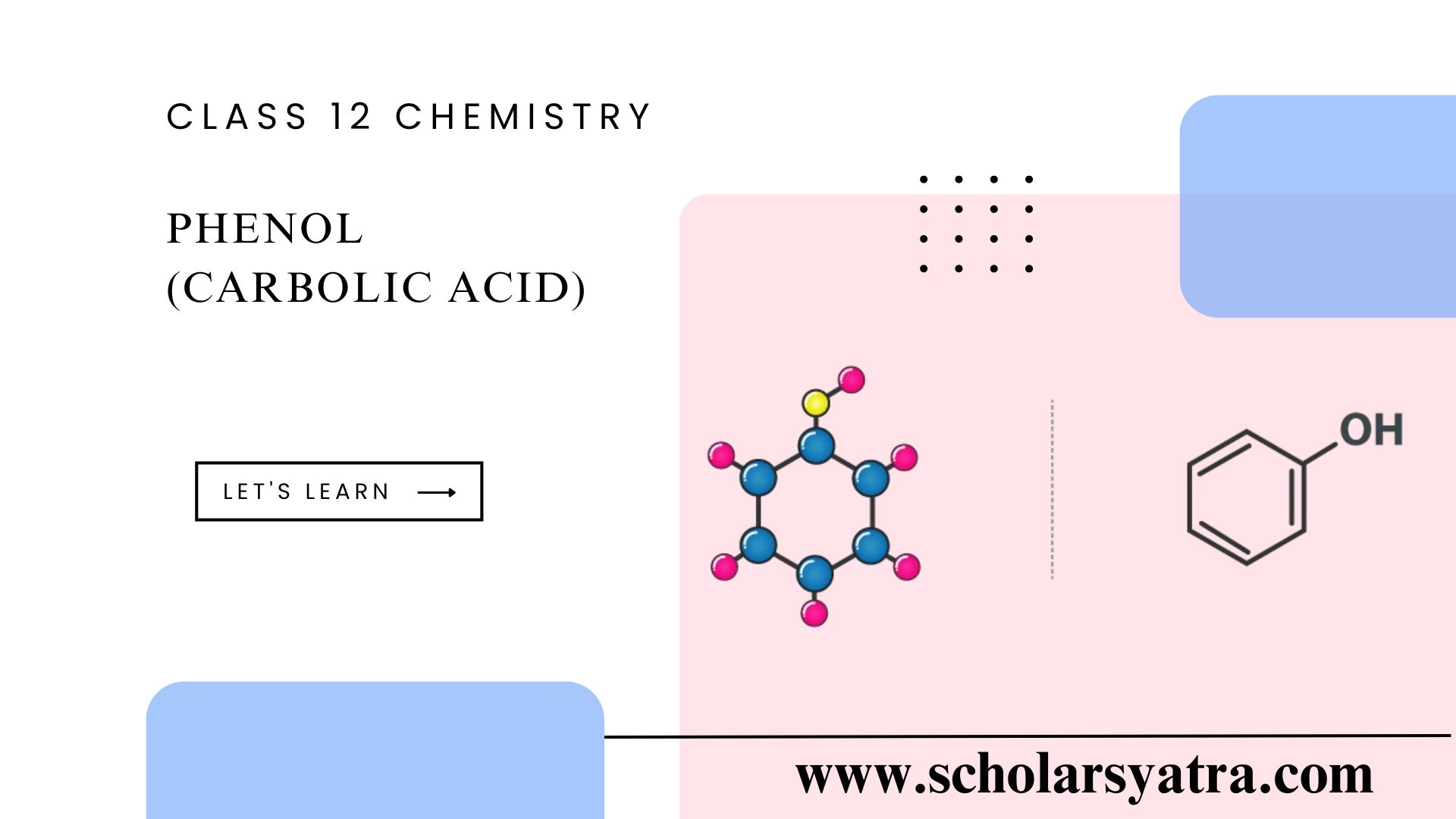
Introduction to Phenol Phenol, also known as carbolic acid, is an organic compound that belongs to the class of aromatic compounds. This aromatic molecule consists
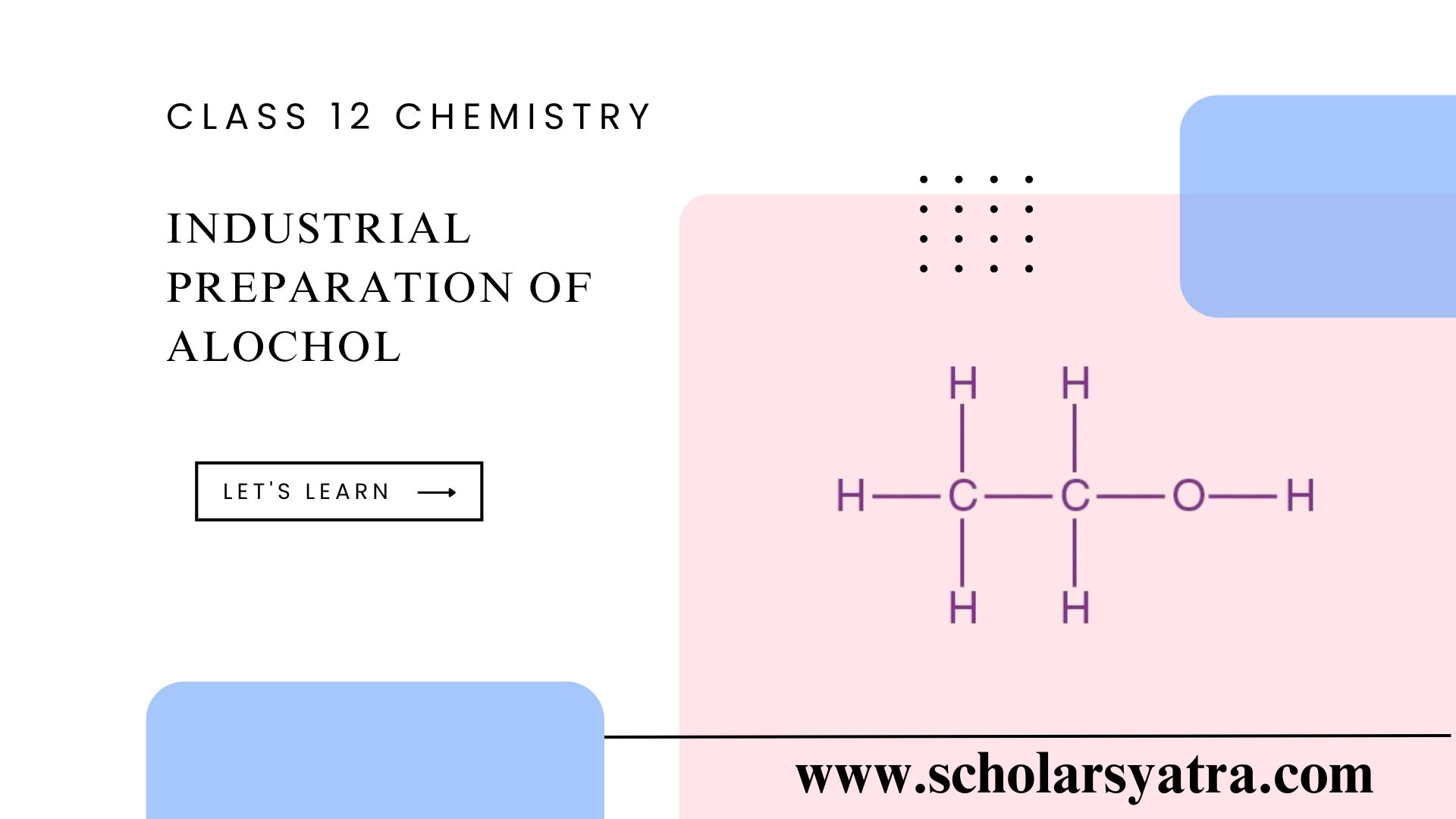
The industrial preparation of alcohol, specifically ethanol (C₂H₅OH), is widely used across various industries, including the beverage, pharmaceutical, and chemical sectors. It is carried out
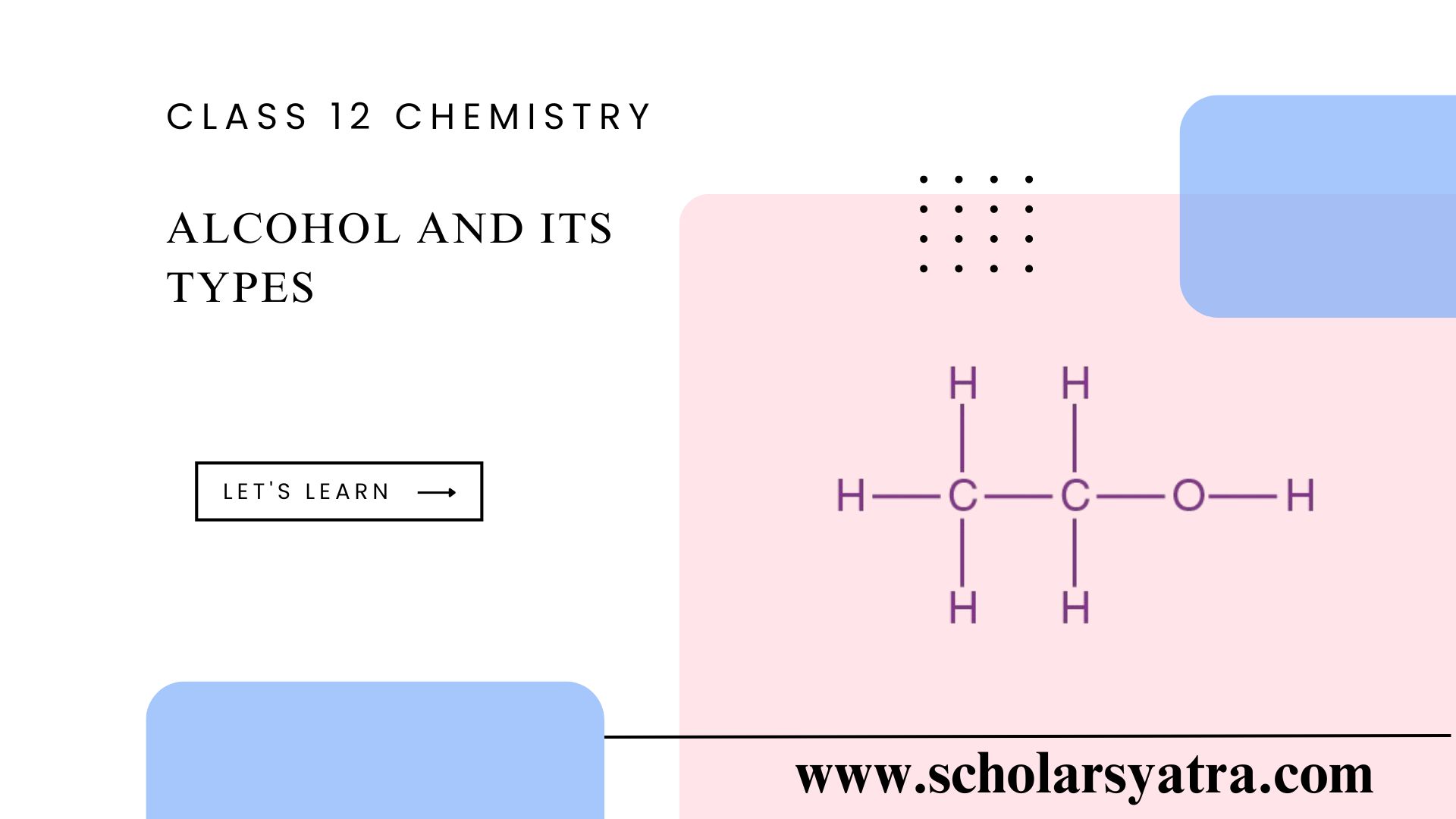
Alcohols are a group of organic compounds characterized by one or more hydroxyl (-OH) groups attached to a carbon atom in a hydrocarbon chain. The
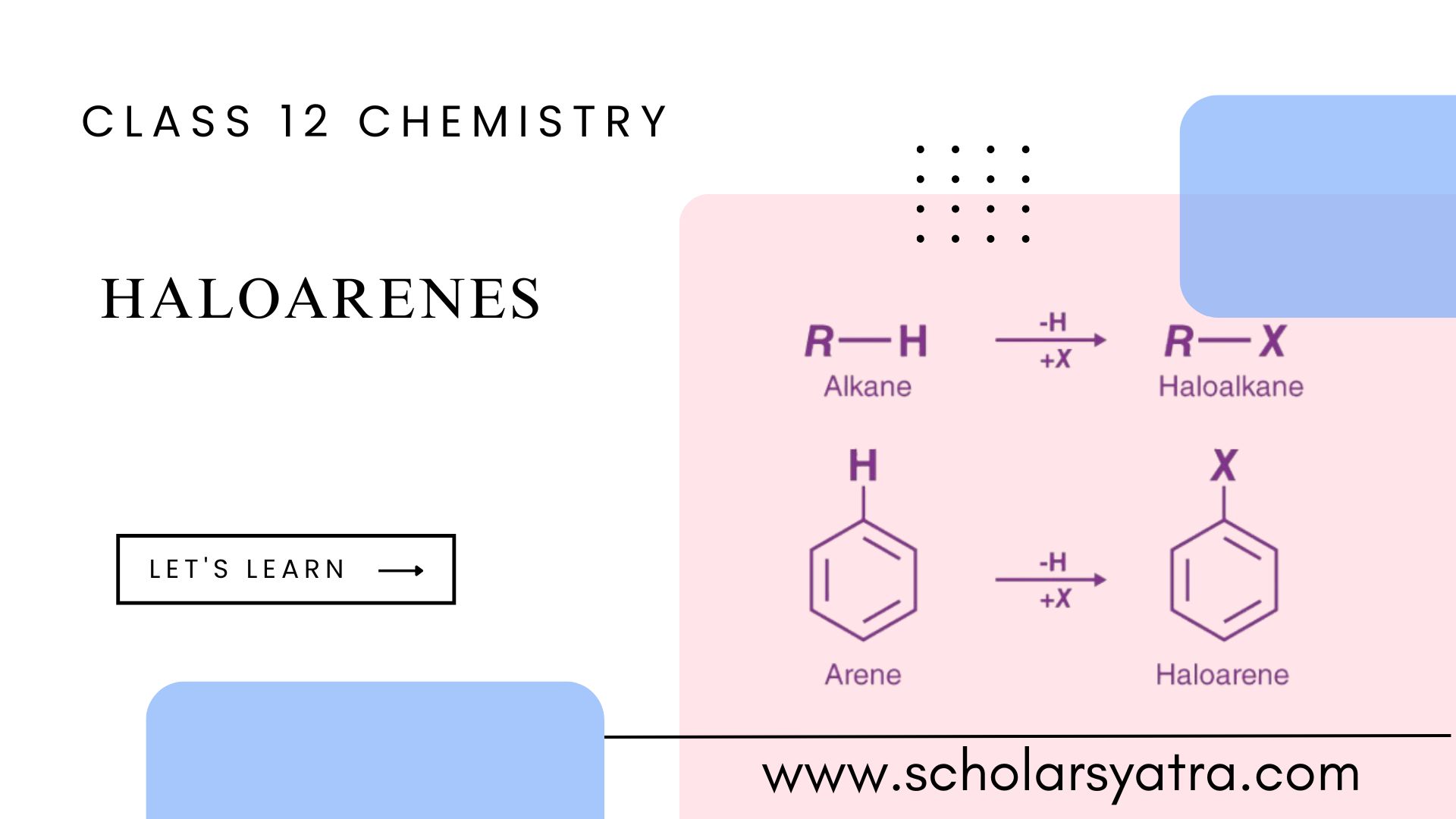
Haloarenes, also known as aryl halides, are aromatic compounds where one or more hydrogen atoms in an aromatic ring (like benzene) are replaced by halogen
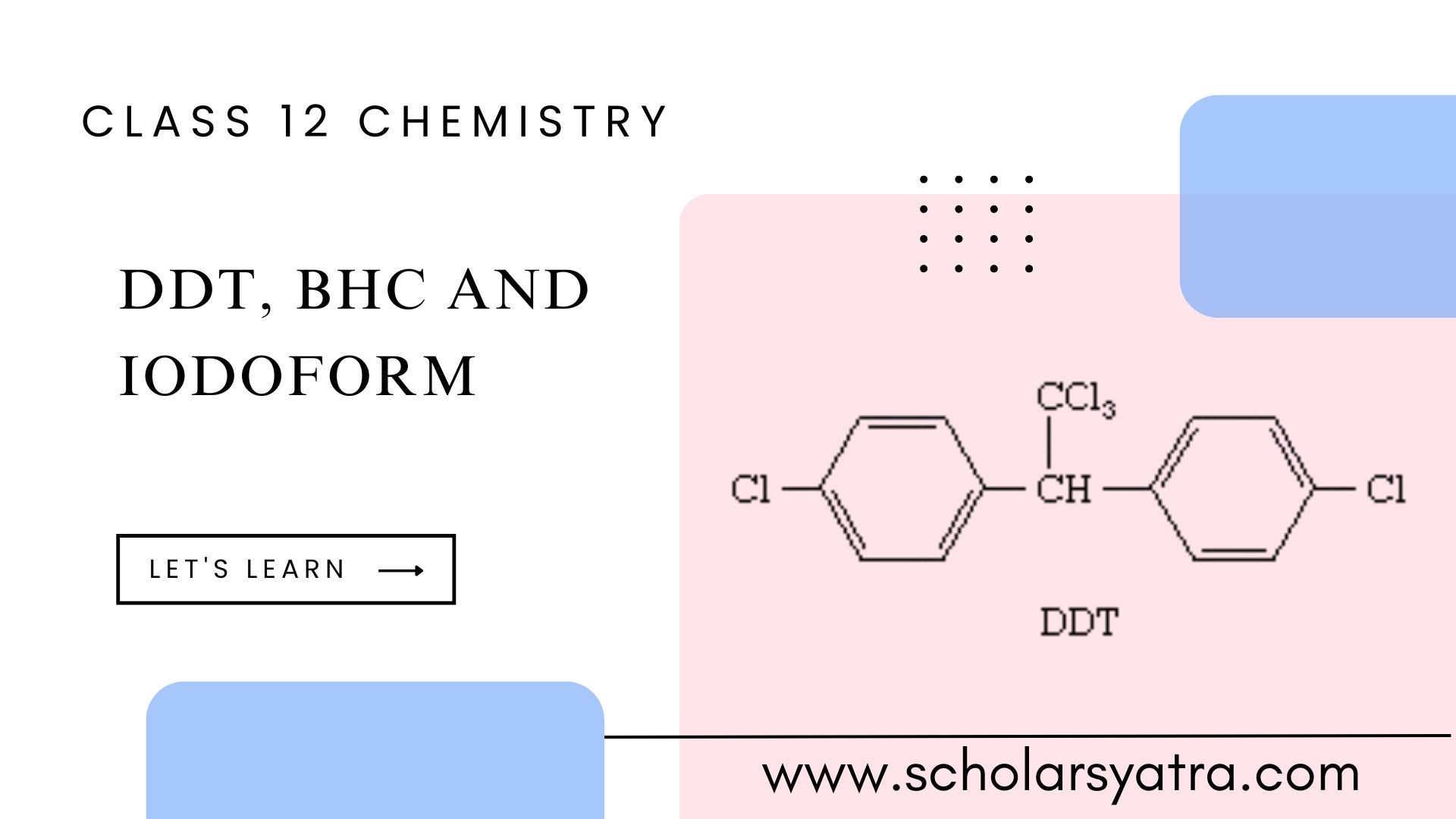
Explore the detailed information on DDT, BHC, and Iodoform, these three topics cover their structure preparation, properties, and uses in daily life. This Learning will
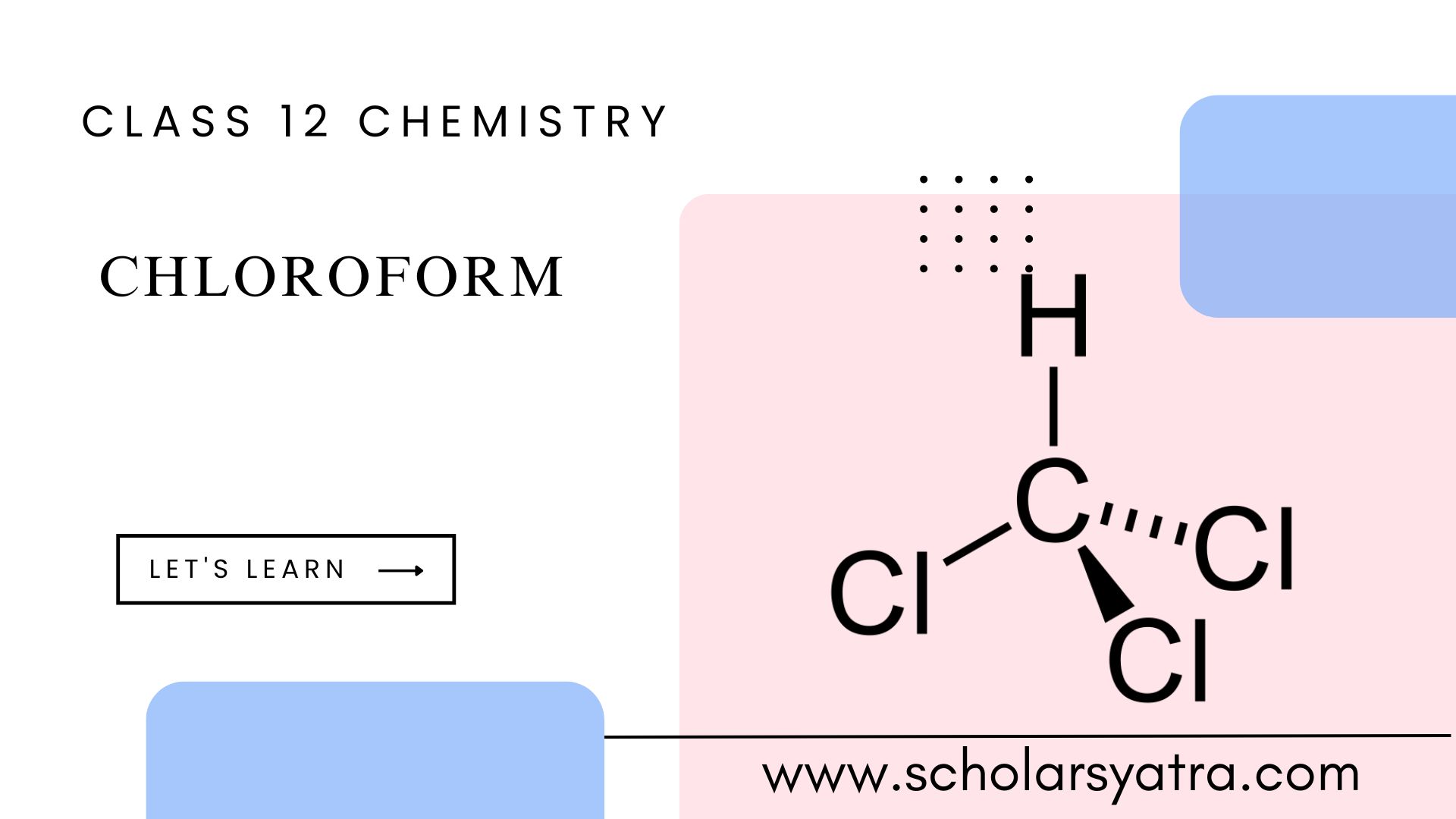
Chloroform is an important chemical compound that has been used for various purposes, such as a solvent and formerly as an anesthetic. Its chemical behavior,
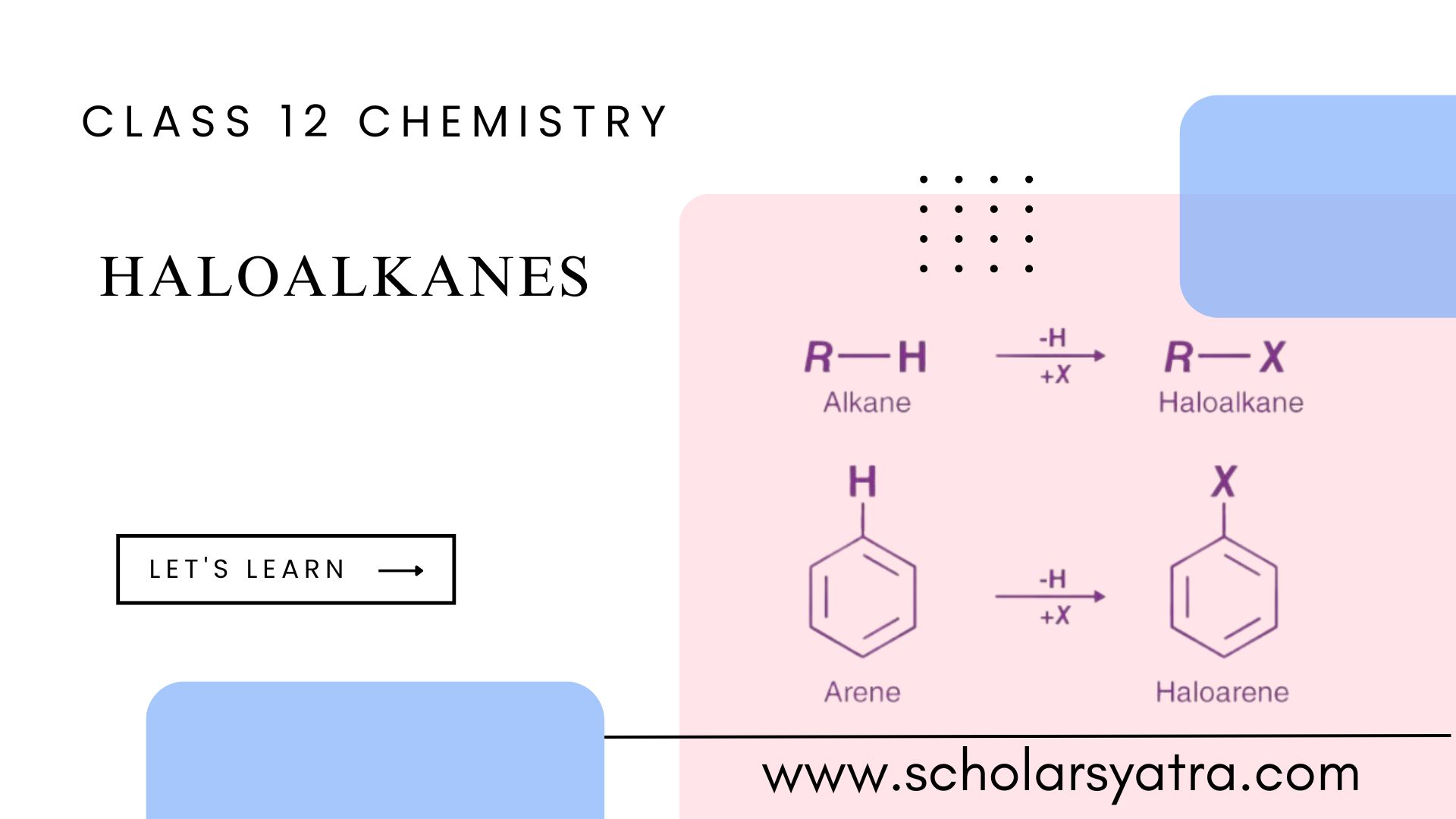
Haloalkane/Haloalkanes, also known as alkyl halides, is a significant class of organic compounds in chemistry. They are derived from alkanes (saturated hydrocarbons) by replacing one
Copyright 2024 | All Rights Reserved by Scholarsyatra