In organic chemistry, detecting elements like nitrogen, sulfur, and halogens (chlorine, bromine, iodine) in compounds is vital for determining molecular composition and structure. The qualitative analysis of these elements is primarily done using specific tests in the lab, and each follows well-established chemical principles.
Table of Contents
ToggleHere’s a guide to understanding the laboratory methods used to detect nitrogen, sulfur, and halogens in organic compounds.
1. Lassaigne’s Test: A General Approach
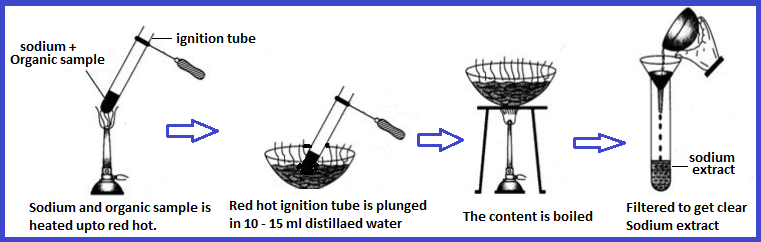
The Lassaigne’s test is one of the most common methods to detect nitrogen, sulfur, and halogens in organic compounds. The principle behind this test is the conversion of covalently bonded nitrogen, sulfur, and halogens into ionic forms, which are easier to detect.
In this test, the organic compound is fused with sodium metal in a fusion tube. During this process, the organic molecule breaks down, and any nitrogen, sulfur, or halogen present is converted into soluble sodium salts of cyanide, sulfide, or halides.
- Nitrogen forms sodium cyanide (NaCN).
- Sulfur forms sodium sulfide (Na₂S).
- Halogens form sodium halides (NaX, where X = Cl, Br, or I).
After the fusion, the fused mass is extracted with water to dissolve these ionic compounds, forming Lassaigne’s extract, which is then used for specific tests to detect the presence of nitrogen, sulfur, and halogens.
2. Detection of Nitrogen
After preparing the Lassaigne extract, the presence of nitrogen is tested by adding freshly prepared ferrous sulfate solution followed by the addition of ferric chloride and heating. After cooling, concentrated sulfuric acid is added.
The reaction produces Prussian blue (ferric ferrocyanide), which confirms the presence of nitrogen.
Reaction:
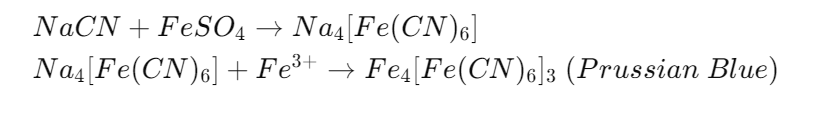
The formation of a blue or green color indicates that nitrogen is present in the organic compound.
3. Detection of Sulfur
Sulfur in the Lassaigne’s extract is detected by two common methods:
a) Lead Acetate Test
To a portion of the extract, add sodium nitroprusside or lead acetate solution. If sulfur is present, black precipitates of lead sulfide (PbS) will form.
Reaction:
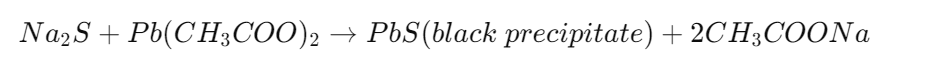
b) Sodium Nitroprusside Test
To another portion of the Lassaigne’s extract, add sodium nitroprusside solution. The appearance of a violet color indicates the presence of sulfur.
4. Detection of Halogens (Chlorine, Bromine, and Iodine)
For the detection of halogens, Lassaigne’s extract is acidified with dilute nitric acid to remove any sulfides or cyanides, which can interfere with the test. Then, silver nitrate solution is added to the acidified extract.
- Chlorine: A white precipitate of silver chloride (AgCl) forms, soluble in ammonia solution.
- Bromine: A pale yellow precipitate of silver bromide (AgBr) forms, sparingly soluble in ammonia.
- Iodine: A yellow silver iodide (AgI) precipitate forms, insoluble in ammonia solution.
Reactions:
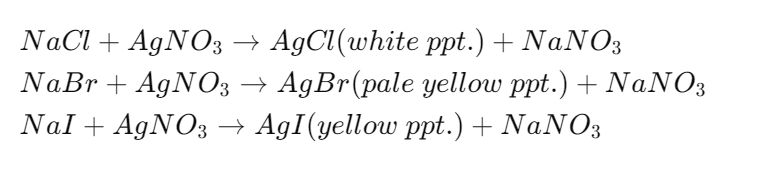
These color changes and solubility patterns help identify which halogen is present in the compound.
5. Precautions and Tips for Accurate Results
- Fusion Temperature: Ensure that the sodium fusion is done at a sufficiently high temperature to completely convert nitrogen, sulfur, and halogens into their ionic forms.
- Avoid Contamination: Use clean apparatus to avoid false positives, especially when testing for halogens, as halide contamination is common.
- Correct Handling of Sodium: Sodium metal is highly reactive, so it should be handled with care. It is typically cut under mineral oil and added in small pieces to prevent hazardous reactions.
- Sufficient Dilution: Make sure the Lassaigne extract is diluted enough to avoid errors in precipitation reactions during halogen detection.
6. Applications of These Tests
- Forensic Analysis: Detection of specific elements in biological or environmental samples.
- Pharmaceutical Chemistry: Testing the composition of organic molecules used in drug development.
- Environmental Chemistry: Identifying pollutants and toxic organic compounds in water or soil samples.
Conclusion
Detecting nitrogen, sulfur, and halogens in organic compounds is a fundamental part of qualitative organic analysis. Lassaigne’s test, coupled with specific precipitation reactions, provides a simple yet powerful method to identify these elements. With careful technique, these methods yield accurate and reliable results, making them indispensable in laboratories across various fields of chemistry.