Alcohols are one of the most well-known and studied classes of organic compounds in chemistry. Characterized by the presence of a hydroxyl group (-OH) attached to a carbon atom, alcohols are versatile compounds with widespread applications in industries, healthcare, and research. This article delves into the chemistry of alcohols, their structures, types, properties, reactions, and their significance in various fields.
Table of Contents
ToggleWhat Are Alcohols?
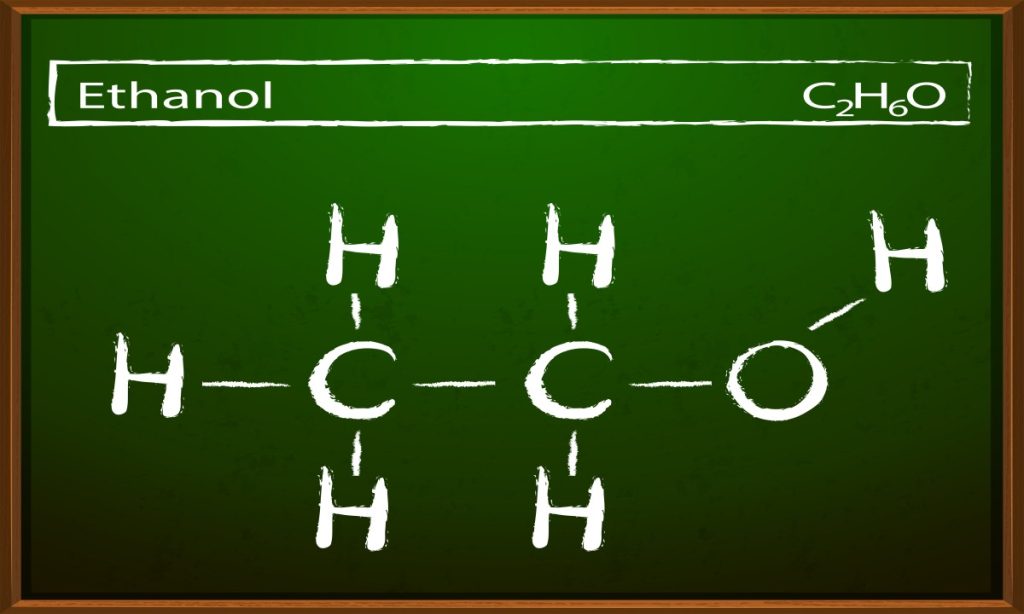
In chemistry, alcohols refer to a broad group of organic compounds that contain one or more hydroxyl (-OH) groups bonded to a saturated carbon atom (sp³ hybridized). The simplest and most common alcohol is ethanol, which is found in alcoholic beverages. The general formula for alcohols is R-OH, where R represents a hydrocarbon group.
Structure of Alcohols
The structure of alcohol consists of two main components:
- Hydrocarbon Chain (R-group): The R-group can vary in size, structure, and complexity, ranging from simple alkyl chains like methyl (CH₃-) to larger, branched, or even aromatic groups.
- Hydroxyl Group (-OH): The hydroxyl group imparts the distinct chemical properties of alcohols, including their ability to form hydrogen bonds and act as weak acids.
Types of Alcohols
Alcohols can be classified based on the number of hydroxyl groups and the carbon atom to which the -OH group is attached:
- Primary Alcohols (1°): The carbon atom attached to the hydroxyl group is connected to only one other carbon atom. Example: Methanol (CH₃OH) and ethanol (C₂H₅OH).
- Secondary Alcohols (2°): The carbon atom attached to the hydroxyl group is connected to two other carbon atoms. Example: Isopropanol (C₃H₇OH).
- Tertiary Alcohols (3°): The carbon atom attached to the hydroxyl group is connected to three other carbon atoms. Example: Tert-butanol (C₄H₉OH).
- Polyols: Alcohols with more than one hydroxyl group. Example: Glycerol (C₃H₅(OH)₃) and ethylene glycol (C₂H₄(OH)₂).
Properties of Alcohols
The chemical and physical properties of alcohols are largely influenced by the hydroxyl group and the nature of the hydrocarbon chain. Some key properties include:
- Polarity and Hydrogen Bonding: Due to the electronegativity of oxygen in the hydroxyl group, alcohols are polar molecules. They can form hydrogen bonds with each other and with water, making lower alcohols like methanol and ethanol highly soluble in water.
- Boiling Points: Alcohols generally have higher boiling points compared to hydrocarbons of similar molecular weight. This is due to the presence of
- bonds between alcohol molecules, which require more energy to break.
- Acidity: Alcohols are weak acids, capable of donating protons (H⁺) from their hydroxyl group. The acidity of alcohol decreases as the number of alkyl groups attached to the carbon atom increases.
- Viscosity: Alcohols, particularly those with longer chains or multiple hydroxyl groups, tend to be more viscous. For example, glycerol is a highly viscous alcohol due to its three hydroxyl groups.
Reactions of Alcohols
Alcohols participate in a wide variety of chemical reactions, making them highly versatile reagents in organic synthesis. Some common reactions include:
- Dehydration: Alcohols can undergo dehydration (loss of water) to form alkenes in the presence of strong acids like sulfuric acid. This reaction is used in industrial processes to produce ethylene from ethanol.
- Oxidation: Alcohols can be oxidized to aldehydes, ketones, or carboxylic acids, depending on the type of alcohol. Primary alcohols are oxidized to aldehydes and further to carboxylic acids, while secondary alcohols are oxidized to ketones. Tertiary alcohols do not undergo oxidation easily.
- Methanol → Formaldehyde (oxidized to formic acid)
- Ethanol → Acetaldehyde (oxidized to acetic acid)
- Esterification: Alcohols react with carboxylic acids to form esters, a reaction catalyzed by acids. Esters are widely used in fragrances, flavorings, and as solvents.
- Substitution Reactions: Alcohols can undergo substitution reactions where the hydroxyl group is replaced by a halogen atom, often using reagents like phosphorus tribromide (PBr₃) or thionyl chloride (SOCl₂).
Industrial and Laboratory Applications of Alcohols
Alcohols are essential chemicals used across multiple industries. Some of their key applications include:
- Solvents: Alcohols like ethanol, methanol, and isopropanol are excellent solvents for a variety of organic and inorganic substances. Their polarity and ability to dissolve both polar and non-polar compounds make them ideal for use in laboratory and industrial settings.
- Fuel and Energy: Ethanol is widely used as a biofuel, either on its own or blended with gasoline to create a cleaner-burning fuel. Methanol is used in some regions as a fuel in internal combustion engines and as a precursor in biodiesel production.
- Pharmaceuticals and Cosmetics: Alcohols serve as important intermediates in the synthesis of pharmaceutical drugs, disinfectants, antiseptics (e.g., isopropyl alcohol), and cosmetic products. Glycerol is a common ingredient in skincare and personal care products due to its moisturizing properties.
- Beverages: Ethanol is the key component of alcoholic drinks like beer, wine, and spirits. In the beverage industry, the fermentation of sugars by yeast produces ethanol, which is consumed in moderate amounts for recreational purposes.
- Chemical Synthesis: Alcohols are versatile building blocks in organic chemistry. They are used to synthesize aldehydes, ketones, esters, and carboxylic acids. Ethanol is commonly used as a reagent in organic reactions.
Health and Safety Considerations
While alcohols have many beneficial uses, some pose health and safety risks. Methanol, for example, is highly toxic and can cause blindness or death if ingested. Ethanol is safer in controlled amounts but can lead to alcohol poisoning if consumed in excess. Proper handling, storage, and alcohol usage are crucial in laboratory and industrial environments.
Summary
Alcohols are fundamental compounds in chemistry, with various applications in everyday life, from solvents and fuels to medical and personal care products. Understanding their structure, properties, and reactions is key to harnessing their full potential in both practical and industrial settings. As we continue to innovate in areas like biofuels and green chemistry, alcohols will remain at the forefront of chemical research and industrial processes.