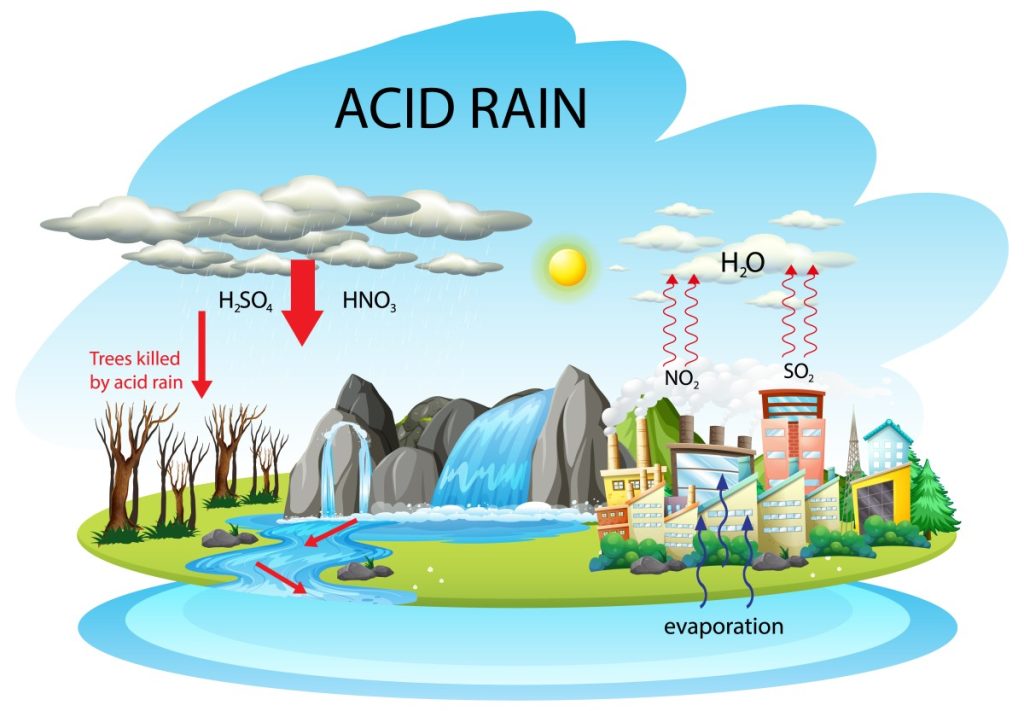
Acid rain refers to any form of precipitation—rain, snow, sleet, or fog—unusually acidic, meaning it contains elevated levels of hydrogen ions (low pH). It is a significant environmental problem that results from human activities, particularly the burning of fossil fuels. Acid rain can devastate natural ecosystems, human health, buildings, and cultural landmarks. This article provides an in-depth exploration of the causes of acid rain, its wide-ranging effects, and the measures that can be taken to control and mitigate its impact.
Table of Contents
ToggleWhat is Acid Rain?
Acid rain is characterized by a pH level below 5.6, which is the average pH of natural, unpolluted rain. Normal rainwater is slightly acidic due to the presence of carbon dioxide (CO₂) in the atmosphere, which dissolves in water to form carbonic acid (H₂CO₃). However, acid rain is more acidic due to the presence of stronger acids such as sulfuric acid (H₂SO₄) and nitric acid (HNO₃), which form from the reaction of water with sulfur dioxide (SO₂) and nitrogen oxides (NOₓ) in the atmosphere.
Causes of Acid Rain
The primary causes of acid rain are human activities and natural sources, with human-induced pollution being the dominant contributor.
1. Emissions of Sulfur Dioxide (SO₂)
- Sources: The combustion of fossil fuels (coal, oil, and natural gas) in power plants, industrial facilities, and vehicles is the largest source of SO₂ emissions. Sulfur is present as an impurity in these fuels, and when burned, it reacts with oxygen in the air to produce sulfur dioxide.
- Chemical Reactions: Once released into the atmosphere, SO₂ reacts with water vapor and oxygen to form sulfuric acid (H₂SO₄):
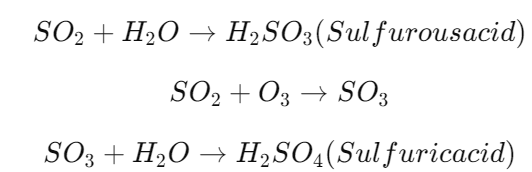
These acids then dissolve in rainwater, leading to acid precipitation.
2. Emissions of Nitrogen Oxides (NOₓ)
- Sources: NOₓ gases are primarily produced from the burning of fossil fuels in vehicles, industrial processes, and power generation. High temperatures in combustion processes facilitate the reaction of nitrogen (N₂) in the air with oxygen (O₂), forming nitrogen oxides like nitrogen dioxide (NO₂) and nitric oxide (NO).
- Chemical Reactions: In the atmosphere, nitrogen oxides undergo a series of reactions to form nitric acid (HNO₃): NO2+OH→HNO3(Nitricacid)NO_2 + OH \rightarrow HNO_3 (Nitric acid)NO2+OH→HNO3(Nitricacid) This nitric acid dissolves in water droplets, contributing to acid rain.
3. Natural Sources
- Volcanic Eruptions: Volcanoes release large quantities of sulfur dioxide and other gases into the atmosphere, which can contribute to acid rain in the vicinity of active volcanic regions.
- Lightning and Forest Fires: Lightning strikes naturally produce small amounts of nitrogen oxides, and forest fires release both sulfur and nitrogen compounds into the atmosphere, albeit on a smaller scale compared to human activities.
- Decaying Vegetation and Oceanic Processes: Some natural processes, such as the decay of organic matter and emissions from oceans, release sulfur compounds into the atmosphere.
Effects of Acid Rain
1. Effects on Ecosystems
a. Aquatic Ecosystems
- Acid rain can severely affect rivers, lakes, and streams by lowering the pH of the water, making it more acidic. Aquatic organisms, particularly fish and amphibians, are highly sensitive to changes in pH levels. When the pH of water drops below 5, many fish species experience difficulties in reproduction, and their populations may decline.
- Acidic water leaches aluminum from the soil into water bodies. Aluminum is toxic to many aquatic organisms and can lead to the death of fish, insects, and other invertebrates. In some cases, entire aquatic ecosystems can collapse due to the loss of biodiversity caused by acid rain.
b. Terrestrial Ecosystems
- Acid rain leaches essential nutrients like calcium, magnesium, and potassium from the soil, which plants need for healthy growth. This nutrient depletion weakens plants, making them more susceptible to diseases, extreme weather conditions, and pests.
- Forests in high-altitude areas are particularly vulnerable to acid rain, as clouds and fog in these regions are often more acidic than rain. Acidic deposition directly damages the leaves and bark of trees, reducing their ability to photosynthesize and grow.
2. Effects on Buildings and Monuments
- Acid rain accelerates the corrosion of buildings, bridges, and monuments, particularly those made of limestone, marble, and concrete. The sulfuric acid in acid rain reacts with calcium carbonate (CaCO₃) in the stone, leading to the formation of calcium sulfate (CaSO₄), which is water-soluble and washes away, gradually eroding the structure. This process is especially damaging to cultural heritage sites like the Taj Mahal in India and the Parthenon in Greece.
3. Effects on Human Health
- While acid rain itself does not pose a direct health risk to humans, the pollutants that cause acid rain—sulfur dioxide and nitrogen oxides—contribute to the formation of fine particulate matter (PM2.5) and ground-level ozone, both of which are harmful to human health. These pollutants can aggravate respiratory conditions such as asthma and bronchitis, and long-term exposure can lead to lung and heart diseases.
- Acidic particles in the air can also irritate the eyes, skin, and mucous membranes, leading to increased cases of eye irritation and skin problems.
4. Effects on Agriculture
- Acid rain can reduce crop yields by depleting soil nutrients and making it harder for plants to absorb essential minerals. Crops like wheat, rice, and maize are sensitive to acidic soils, which can stunt their growth and reduce productivity.
- Furthermore, acid rain damages the waxy protective coatings on plant leaves, making them more susceptible to disease and pests.
Controlling Measures for Acid Rain
1. Reducing Sulfur Dioxide (SO₂) and Nitrogen Oxides (NOₓ) Emissions
- Switching to Cleaner Energy Sources: Reducing reliance on fossil fuels and promoting the use of renewable energy sources like wind, solar, and hydropower can significantly decrease the emissions of SO₂ and NOₓ. Nuclear energy is another cleaner alternative, though it comes with its own set of challenges.
- Use of Scrubbers in Industrial Plants: Power plants and industrial facilities can install scrubbers, which are devices that remove sulfur dioxide from exhaust gases before they are released into the atmosphere. Wet scrubbers, for example, use a slurry of limestone to react with sulfur dioxide, preventing its release.
- Catalytic Converters in Vehicles: Catalytic converters installed in automobiles reduce nitrogen oxide emissions by converting them into less harmful nitrogen (N₂) and oxygen (O₂). This technology is mandatory in many countries and has proven effective in reducing vehicle emissions.
2. Regulations and Policies
- Clean Air Acts and Emission Limits: Many countries have implemented strict air quality standards to limit the emissions of sulfur dioxide and nitrogen oxides. The United States, for example, passed the Clean Air Act in 1970, and amendments in the 1990s specifically targeted acid rain through a cap-and-trade system for sulfur dioxide emissions.
- International Agreements: Since air pollution and acid rain are transboundary issues, international cooperation is essential. The Gothenburg Protocol to the Convention on Long-Range Transboundary Air Pollution (CLRTAP) is one such agreement aimed at reducing the emissions of sulfur and nitrogen compounds across Europe and North America.
3. Liming of Acidified Lakes and Soils
- Liming involves adding limestone (calcium carbonate) or other alkaline substances to lakes, rivers, and soil to neutralize their acidity. This is a temporary but effective measure to restore ecosystems that have been damaged by acid rain. Liming increases the pH of the water or soil, making it less acidic and more hospitable to aquatic life and plant growth.
4. Public Awareness and Individual Actions
- Energy Conservation: Reducing energy consumption can lower the demand for electricity generated from fossil fuels. Individuals can conserve energy by using energy-efficient appliances, turning off lights when not in use, and utilizing public transportation or carpooling.
- Alternative Transportation: Encouraging the use of electric vehicles, bicycles, and public transport can reduce the number of vehicles on the road and, consequently, nitrogen oxide emissions.
Conclusion
Acid rain is a complex environmental issue with wide-ranging effects on ecosystems, infrastructure, human health, and agriculture. Its primary causes—sulfur dioxide and nitrogen oxide emissions—stem from human activities, particularly the burning of fossil fuels. While acid rain continues to pose a serious threat, effective controlling measures such as reducing emissions, enforcing air quality regulations, and adopting clean energy sources have shown significant progress in mitigating its impact. Further global cooperation and the adoption of sustainable practices are essential to combating acid rain and preserving both the environment and public health.