Introduction to Phenol
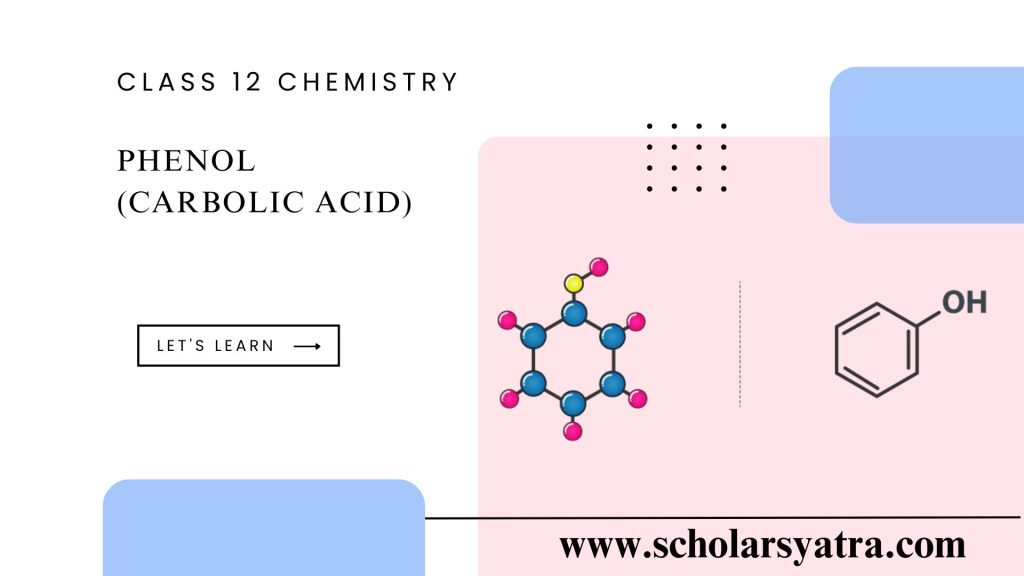
Phenol, also known as carbolic acid, is an organic compound that belongs to the class of aromatic compounds. This aromatic molecule consists of a hydroxyl group (-OH) bonded to a benzene ring. Its chemical formula is C₆H₅OH. Phenol is a white crystalline solid that is slightly soluble in water and has a distinctive odor. Its unique structure and properties make it essential in various chemical processes and industries, including pharmaceuticals, resins, and antiseptics.
Table of Contents
ToggleFriedlieb Ferdinand Runge discovered Phenol in the year 1834. It was extracted from coal tar and is also known as phenolic acid. If a compound consists of a six-membered aromatic ring and is directly bonded to a hydroxyl group, then it can be referred to as phenol.
Structure of Phenol (Carbolic acid)- C6H6O
The structure of phenol is characterized by a hydroxyl (-OH) group attached directly to a benzene ring. This configuration makes phenol an aromatic alcohol, but it behaves quite differently from other alcohols. Due to the conjugation of the benzene ring with the hydroxyl group, phenol exhibits special chemical reactivity and physical properties.
- Molecular Formula: C₆H₅OH
- Molecular Structure: A six-membered benzene ring attached to one hydroxyl group
- Bonding: The -OH group’s lone pairs are partially involved in resonance with the benzene ring, leading to increased stability.
Nomenclature of Phenol (Carbolic acid)
Phenols are organic compounds containing at least one -OH group directly attached to the benzene ring. Depending upon the number of hydroxyl groups attached to the benzene ring, phenols/carbolic acid can be classified as monohydric, dihydric, and trihydric phenols.
- Monohydric phenols – The simplest member of the series is hydroxybenzene, commonly known as phenol, while others are named substituted phenols. The three isomeric hydroxyl toluenes are known as cresols.
- Dihydric phenols – The three isomeric dihydroxy benzenes namely catechol, resorcinol, and quinol are better known by their common names.
- Trihydric phenols – Trihydroxy phenols are known by the common names called pyrogallol, hydroxyquinol, and phloroglucinol.
Physical Properties of Phenol (Carbolic acid)- C6H6O
Phenol has distinct physical properties that make it easily identifiable:
| C6H6O | Phenol |
| Molecular Weight/ Molar Mass | 94.11 g/mol |
| Density | 1.07 g/cm³ |
| Melting Point | 40.5 °C |
| Boiling Point | 181.7 °C |
Chemical Properties of Phenol (Carbolic acid)
Phenol exhibits a range of chemical reactions due to its unique structure. The benzene ring influences the -OH group, making phenol more reactive than typical alcohols. Some of the significant reactions of phenol include:
1. Acidity of Phenol
Phenol is weakly acidic, which is unusual for an alcohol. It can donate a proton (H⁺) from the hydroxyl group, forming a phenoxide ion (C₆H₅O⁻). This acidity is attributed to the resonance stabilization of the phenoxide ion.
- Reaction with Bases: Phenol reacts with strong bases like sodium hydroxide (NaOH) to form sodium phenoxide and water.C₆H₅OH + NaOH → C₆H₅ONa + H₂O
2. Electrophilic Substitution Reactions
Due to the activating effect of the -OH group, the benzene ring in phenol is highly reactive toward electrophilic substitution reactions. Common substitution reactions include:
- Nitration: Phenol reacts with dilute nitric acid (HNO₃) to form ortho and para nitrophenol.C₆H₅OH + HNO₃ → C₆H₄(NO₂)OH (o- and p- nitrophenol)
- Halogenation: When phenol reacts with bromine water, it forms 2,4,6-tribromophenol as a white precipitate.C₆H₅OH + 3Br₂ → C₆H₂Br₃OH + 3HBr
3. Oxidation
Under controlled conditions, phenol can undergo oxidation reactions. Strong oxidizing agents can oxidize phenol to produce benzoquinone.
Synthesis of Phenol (Carbolic acid)- C6H6O
Phenols (carbolic acid) can be synthesized by the following methods:
1. From sulphonic acids (by alkali fusion of sodium benzene sulphonate)
The first commercial process for the synthesis of phenol. Sodium benzene sulphonate is fused with sodium hydroxide at 573K to produce sodium phenoxide, which upon acidification yields phenol.

Synthesis of Phenols From Sulphonic Acids
2. From diazonium salts (by the hydrolysis of diazonium salt – laboratory method)
When a diazonium salt solution is steam distilled or added to boiling dil.H2SO4, it forms phenol.

Synthesis of Phenols From Diazonium Salts
Chemical Reactions of Phenol (Carbolic acid)- C6H6O
A hydroxyl group is attached to an aromatic ring and it is strongly activating ortho/para director, phenols possess considerable reactivity at their ortho and para carbons toward electrophilic aromatic substitution.
1. Reactions of the Aromatic Ring
The -OH group in phenol is ortho and para directing because it increases electron density at ortho and para positions due to resonance. Thus phenol undergoes electrophilic substitution reactions.
2. Halogenation
Like -NH2 group, -OH group is so activating that it is rather difficult to prevent poly substitution.
If it is required to arrest the reaction at the mono substitution stage, the reaction should be carried out in non-polar solvents like CCl4 and CS2 and at lower temperatures.
5 Uses of Phenol (Carbolic Acid)
Phenol has a broad range of applications across various industries, here the major 5 uses of phenol are given:
- Antiseptics: Phenol has antimicrobial properties and was one of the first antiseptics used in medical practice.
- Pharmaceuticals: Used in the synthesis of aspirin and other drugs.
- Plastic Production: An essential ingredient in the manufacture of phenolic resins, such as Bakelite.
- Dyes and Explosives: Used in dye production and as a precursor for making picric acid, an explosive compound.
- Laboratory Reagent: In research and medical labs, phenol is used in DNA extraction procedures. Its properties help separate nucleic acids from proteins in samples, aiding in genetic and molecular biology studies.
Health and Safety Concerns of Carbolic Acid
Phenol (Carbolic Acid) is toxic and can cause severe burns upon contact with the skin. Inhalation or ingestion can be hazardous, so proper safety measures are essential when handling phenol. Safety protocols include wearing protective gloves, and masks, and working in well-ventilated spaces.