Alcohols are a group of organic compounds characterized by one or more hydroxyl (-OH) groups attached to a carbon atom in a hydrocarbon chain. The general formula for an alcohol is R-OH, where “R” represents an alkyl group (a chain of carbon and hydrogen atoms). They are used as sweeteners and in making perfumes, are valuable intermediates in synthesizing other compounds, and are among the industry’s most abundantly produced organic chemicals.
Table of Contents
ToggleGeneral Structure of Alcohol
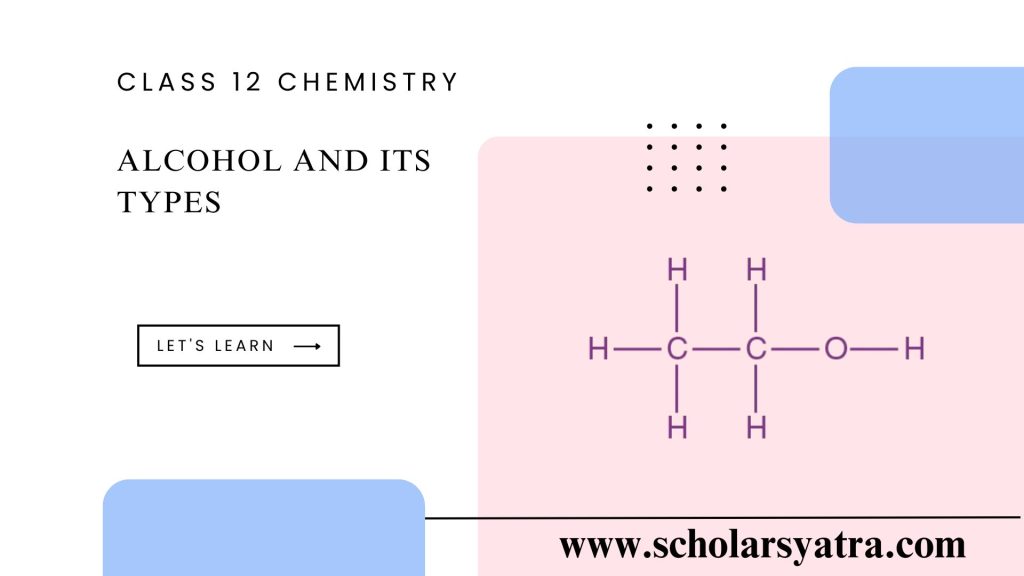
The structure of alcohol revolves around the hydroxyl group (-OH), which is bonded to a carbon atom within the hydrocarbon chain. This hydroxyl group distinguishes alcohols from other organic compounds and contributes to alcohol’s polar nature, hydrogen bonding capability, and solubility in water.
The position and arrangement of the hydroxyl group can vary, which impacts the properties and classification of the alcohol. In the simplest form, methanol (CH₃OH) has a single carbon bonded to the hydroxyl group, whereas ethanol (C₂H₅OH) has a two-carbon chain with a hydroxyl group.
Classification of Alcohol in Chemistry
Alcohols can be classified in various ways based on structure, the number of hydroxyl groups, and the nature of the carbon atom bonded to the -OH group.
1. Classification Based on Hydroxyl Groups
- Monohydric Alcohols: Contain only one hydroxyl group, such as ethanol (C₂H₅OH) and methanol (CH₃OH).
- Dihydric Alcohols (Glycols): Contain two hydroxyl groups, such as ethylene glycol (C₂H₄(OH)₂).
- Trihydric Alcohols: Contain three hydroxyl groups, such as glycerol (C₃H₅(OH)₃).
- Polyhydric Alcohols: Alcohols with more than three hydroxyl groups, such as sorbitol (C₆H₁₄O₆).
2. Classification Based on Carbon Bonding (Primary, Secondary, Tertiary)
- Primary (1°) Alcohols: The carbon with the -OH group is attached to only one other carbon atom. Example: ethanol (C₂H₅OH).
- Secondary (2°) Alcohols: The carbon with the -OH group is attached to two other carbons. Example: isopropanol (C₃H₇OH).
- Tertiary (3°) Alcohols: The carbon with the -OH group is attached to three other carbon atoms. Example: tert-butanol (C₄H₉OH).
3. Classification Based on Saturation
- Saturated Alcohols: Contain only single bonds between carbon atoms.
- Unsaturated Alcohols: Contain one or more double or triple bonds within the carbon chain, such as allyl alcohol (CH₂=CHCH₂OH).
Properties of Alcohol
Alcohols exhibit a range of physical and chemical properties due to the presence of the hydroxyl group. Here are some of the key properties:
Physical Properties of Alcohol
- Boiling Point: Alcohols generally have higher boiling points than hydrocarbons of similar molecular weight due to hydrogen bonding between -OH groups.
- Solubility: Lower alcohols (methanol, ethanol, and propanol) are highly soluble in water because of hydrogen bonding. However, solubility decreases as the carbon chain length increases.
- Polarity: Alcohols are polar due to the hydroxyl group, which makes them excellent solvents for polar and ionic substances.
- Odor and Taste: Alcohols usually have distinct odors and, in the case of ethanol, a slightly sweet taste. Higher alcohols may have unpleasant or burning odors.
Chemical Properties of Alcohol
- Acidity: Alcohols are slightly acidic, with a pKa value around 15-19. They can lose the hydrogen from the -OH group to form alkoxide ions (R-O⁻).
- Oxidation: Primary alcohols oxidize to form aldehydes and, with further oxidation, carboxylic acids. Secondary alcohols oxidize to ketones, while tertiary alcohols generally do not oxidize easily.
- Dehydration: When heated with an acid catalyst, alcohols undergo dehydration to form alkenes.
- Reaction with Metals: Alcohols react with reactive metals, like sodium or potassium, to produce alkoxides and hydrogen gas.
Preparation of Alcohol
Alcohols can be prepared through several methods, depending on the type of alcohol and desired application. Here are some common methods of alcohol preparation:
1. Fermentation
Fermentation is a biological process used to produce ethanol, primarily for beverages and fuel. In this process, yeast converts sugars (from fruits or grains) into ethanol and carbon dioxide:
C6H12O6→2C2H5OH+2CO2
2. Hydration of Alkenes
Alkenes can be hydrated to produce alcohol. This process, known as catalytic hydration, involves adding water (H₂O) across the double bond in the presence of an acid catalyst:
CH2=CH2+H2O→CH3CH2OH
For example, ethene can be hydrated to produce ethanol.
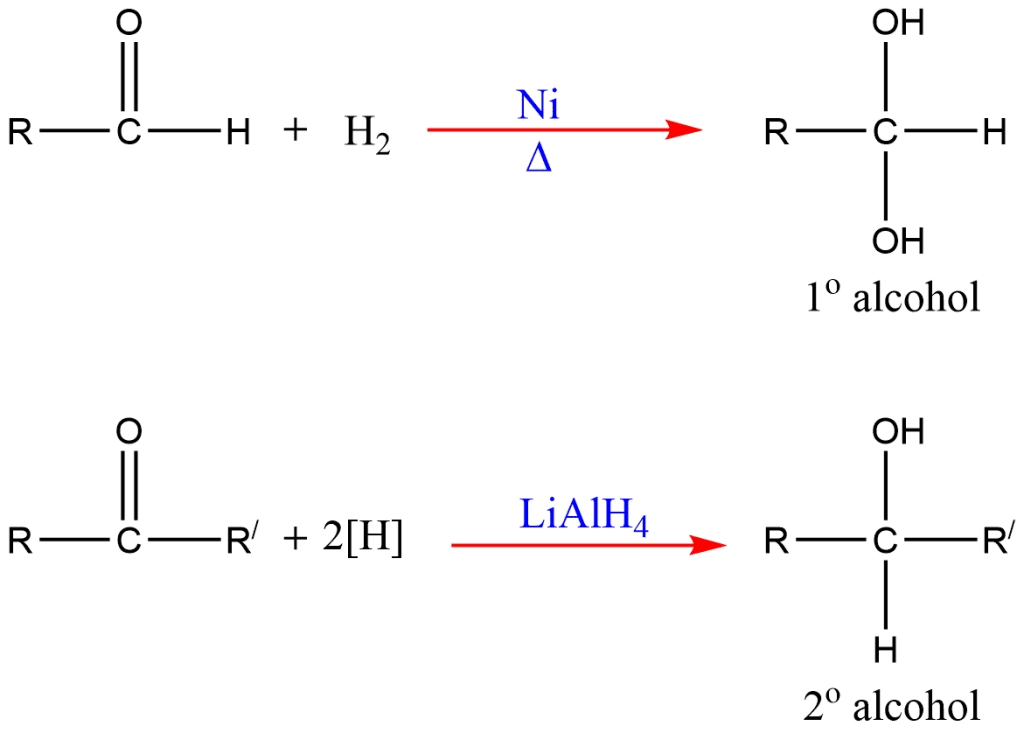
3. Reduction of Aldehydes and Ketones
Aldehydes and ketones can be reduced to form primary and secondary alcohols, respectively. Common reducing agents include lithium aluminum hydride (LiAlH₄) and sodium borohydride (NaBH₄):
R-CHO+H2→R-CH2OH
On reduction, aldehyde gives primary alcohols and ketone gives secondary alcohols.
4. Grignard Reaction
The Grignard reaction involves the reaction of a Grignard reagent (R-MgX) with a carbonyl compound to form an alcohol. This reaction is particularly useful in synthesizing complex alcohols:
R-MgX+R’CHO→R-CH(OH)-R’
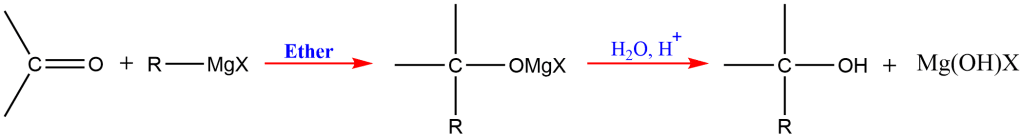
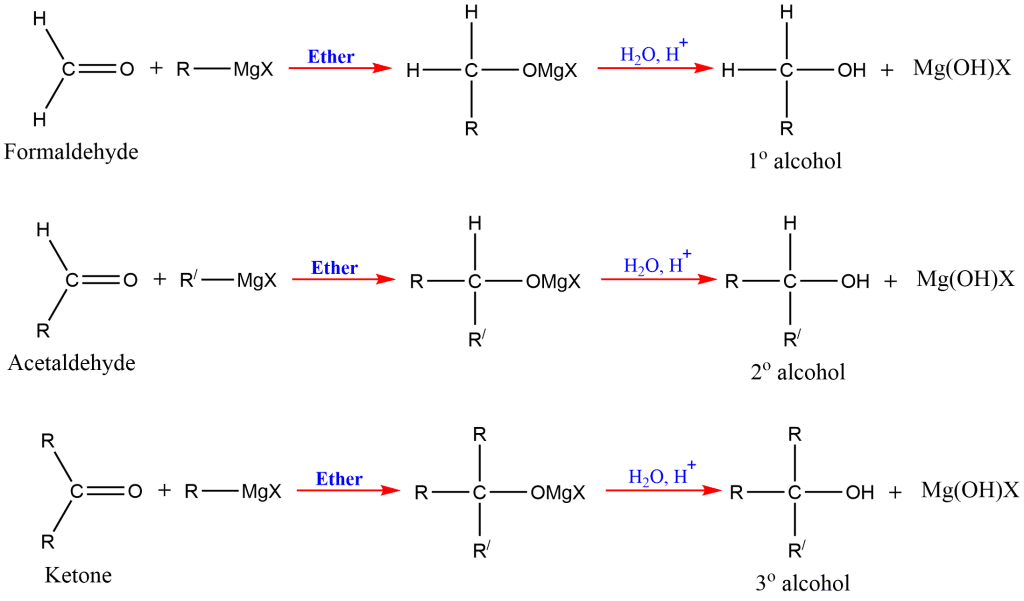
The reaction of Grignard reagents with formaldehyde gives primary alcohols, while the aldehydes other than formaldehyde give secondary alcohol. Likewise, Ketones give tertiary alcohol in reaction with this reagent.
5. Hydrolysis of Alkyl Halides
Alcohols can also be prepared by hydrolyzing alkyl halides (R-X) in the presence of a base. For example:
R-X+OH−→R-OH+X−
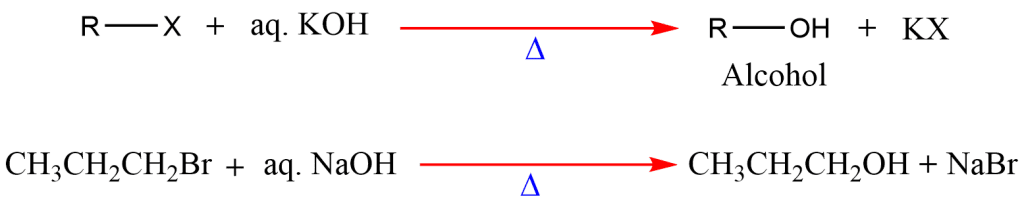
It is not a particularly effective method of preparing alcohols because the tertiary alkyl halides undergo a competitive elimination that results in the undesirable side reaction yielding alkene.
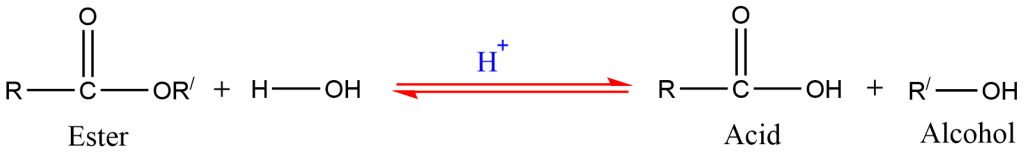
6. Hydrolysis of ester
Alcohols are prepared by the hydrolysis of ester in the presence of acid.
7. From Primary amines
Aliphatic primary amines in reaction with nitrous acid (NaNO2 + HCl) at 0oC-10oC temperature give alcohol.
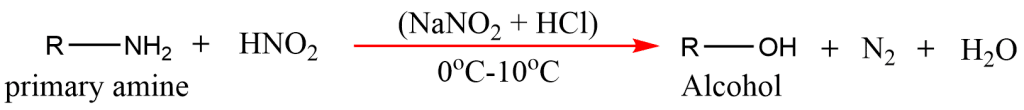
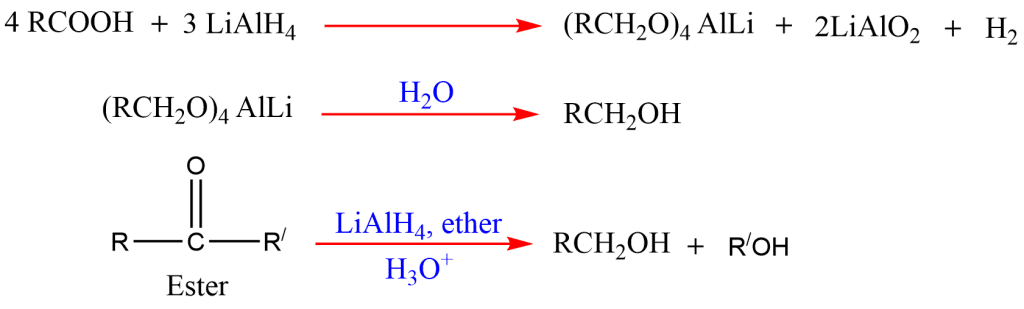
8. Reduction of acid and esters
Alcohol can be prepared by the reduction of carboxylic acids and esters with reducing agents, lithium aluminum hydride.
9. Oxymercuration-Demercuration of alkene
It is one of the most important methods for the preparation of alcohol. Alkene reacts with mercuric acetate in a mixture of tetrahydrofuran (THF) and water to form hydroxy alkyl mercuric compounds, which on reduction with sodium borohydride yields alcohol. The mechanism involves Markonikov’s rule of the addition of water to the Carbon-carbon double bond.

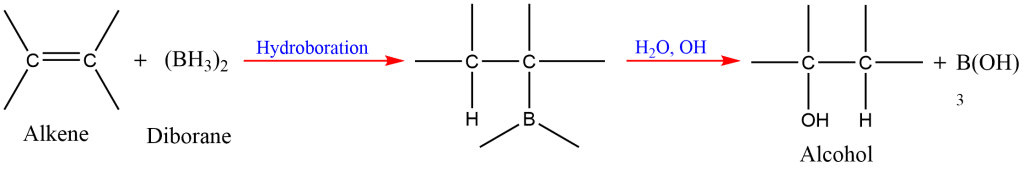
9. Hydroboration-Oxidation of Alkene
Alkene reacts with diborane to form trialkylboranes, which on oxidation with alkaline hydrogen peroxide form alcohols.
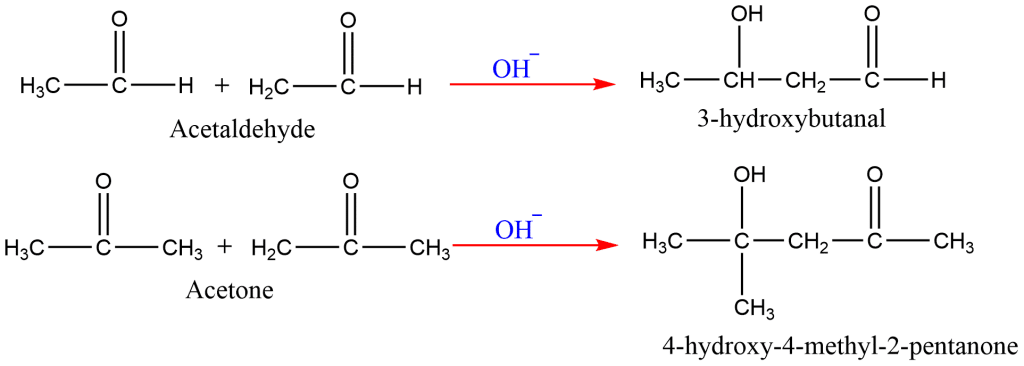
10. Aldol Condensation
In the presence of a base, two molecules of acetaldehyde or ketone containing α-hydrogens combine to form a β-hydroxyaldehyde or β-hydroxyketone.
Applications of Alcohols
Due to their diverse properties, alcohols have a wide range of applications:
- Ethanol: Used in alcoholic beverages, as a fuel additive, in hand sanitizers, and as a solvent.
- Methanol: Used as a fuel, antifreeze, and industrial solvent.
- Isopropyl Alcohol: Commonly used as a disinfectant and in cleaning products.
- Glycerol: Used in pharmaceuticals, cosmetics, and as a food additive.