Haloalkane/Haloalkanes, also known as alkyl halides, is a significant class of organic compounds in chemistry. They are derived from alkanes (saturated hydrocarbons) by replacing one or more hydrogen atoms with a halogen atom. The study of haloalkanes is essential due to their presence in various chemical processes, synthetic applications, and industrial uses.
Table of Contents
ToggleWhat is a Haloalkane?
A haloalkane is an organic compound that contains at least one halogen (fluorine, chlorine, bromine, or iodine) atom attached to a carbon atom in an alkane chain. The general formula for a mono-haloalkane (where only one hydrogen atom is replaced) can be represented as:
R–X
where:
- RRR = alkyl group (e.g., methyl, ethyl, propyl)
- XXX = halogen atom (e.g., F, Cl, Br, I)
Classification of Haloalkanes (Alkyl Halide)
Haloalkanes can be classified based on different criteria, including the number of halogen atoms present, the nature of the carbon atom attached to the halogen, and the type of halogen atom involved.
Classification based on the number of Halogen Atoms
This classification divides haloalkanes into 3 categories based on how many hydrogen atoms in the alkane have been replaced by halogen atoms.
- Monohaloalkanes: Compounds with only one halogen atom attached to the carbon chain.
- Example: Chloromethane(CH3Cl) – A simple haloalkane where one hydrogen of methane (CH4) is replaced by a chlorine atom.
- Example: Bromoethane (C2H5Br) – A two-carbon alkane with one bromine atom attached.
- Dihaloalkanes: Compounds containing two halogen atoms. Dihaloalkanes can be further categorized based on the position of the halogens:
- Geminal Dihaloalkanes: Both halogen atoms are attached to the same carbon atom.
- Example: 1,1-Dichloroethane (CH3CHCl2) – Both chlorine atoms are bonded to the first carbon of an ethane molecule.
- Vicinal Dihaloalkanes: Halogen atoms are attached to adjacent carbon atoms.
- Example: 1,2-Dibromoethane (CH2BrCH2Br) – Bromine atoms are bonded to the first and second carbon of the ethane chain.
- Geminal Dihaloalkanes: Both halogen atoms are attached to the same carbon atom.
- Polyhaloalkanes: Compounds containing more than two halogen atoms.
- Example: Chloroform (CHCl3) – Three chlorine atoms attached to a single carbon atom.
- Example: Carbon tetrachloride (CCl4) – A carbon atom fully substituted with four chlorine atoms.
Classification based on the Nature of the Carbon Atom
Haloalkane is classified based on whether the carbon atom bonded to the halogen is primary, secondary, or tertiary.
- Primary (1°) Haloalkanes: The carbon atom attached to the halogen is connected to only one carbon atom (or part of a methyl group with no other carbon attached).
- Example: 1-Chloropropane (CH3CH2CH2Cl) – The chlorine atom is bonded to the end carbon in the three-carbon chain.
- Example: Bromomethane (CH3Br) – A single carbon bonded directly to bromine with no other carbon attached.
- Secondary (2°) Haloalkanes: The carbon atom attached to the halogen is connected to two other carbon atoms.
- Example: 2-Chloropropane (CH3CHClCH3) – A three-carbon chain bonds The chlorine atom to the middle carbon.
- Example: 2-Bromobutane (CH3CHBrCH2CH3) – The bromine atom is bonded to the second carbon in the four-carbon chain.
- Tertiary (3°) Haloalkanes: The carbon atom attached to the halogen is connected to three other carbon atoms.
- Example: 2-Bromo-2-methylpropane ((CH3)3CBr) – The bromine atom is attached to a carbon that is connected to three methyl groups.
- Example: 2-Chloro-2-methylbutane ((CH3)2C(Cl)CH2CH3) – The chlorine atom is attached to a tertiary carbon in a butane chain.
Classification based on the type of Halogen Atom
Haloalkane is classified based on the type of halogen atom present in the molecule. This classification affects the reactivity and physical properties of the compound.
- Fluoroalkanes: Compounds containing fluorine atoms.
- Example: Fluoromethane (CH3F) – A methyl group with one fluorine atom attached.
- Chloroalkanes: Compounds containing chlorine atoms.
- Example: Chloroethane (C2H5Cl) – Ethane with one hydrogen replaced by chlorine.
- Bromoalkanes: Compounds containing bromine atoms.
- Example: Bromopropane (CH3CH2CH2Br) – Propane with a bromine atom on the first carbon.
- Iodoalkanes: Compounds containing iodine atoms.
- Example: Iodomethane (CH3I) – A single carbon atom attached to iodine.
Multiple Substitutions (Mixed Halides)
Some haloalkanes have more than one type of halogen atom attached to the carbon chain, known as mixed halides.
- Example: Chlorofluoromethane (CH2ClF) – A methane molecule with one chlorine and one fluorine atom attached.
- Example: Bromochloromethane (CH2BrCl) – A methane molecule with one bromine and one chlorine atom attached.
Summary Table
| Type | Structure Example | Example Name |
| Monohaloalkane | CH3Cl | Chloromethane |
| Dihaloalkane (Geminal) | CH3CHCl2 | 1,1-Dichloroethane |
| Dihaloalkane (Vicinal) | CH2ClCH2Cl | 1,2-Dichloroethane |
| Primary Haloalkane | CH3CH2Cl | 1-Chloroethane |
| Secondary Haloalkane | CH3CHClCH3 | 2-Chloropropane |
| Tertiary Haloalkane | (CH3)3CBr | 2-Bromo-2-methylpropane |
| Fluoroalkane | CH3F | Fluoromethane |
| Iodoalkane | CH3I | Iodomethane |
Nomenclature of haloalkanes
A. Common system
- In the common system, haloalkanes are named by adding the word halide after the name of the alkyl group.
- The words n-, sec-, tert-, iso-, and neo- are usually used in writing the common names.
Common name of alkyl halide is always written as two separate words.
B. IUPAC system
- In the IUPAC system, haloalkanes are named by adding the prefix ‘halo-‘ before the name of the parent alkane.
The IUPAC name of any monohalo alkanes is always written as one word.

Note : n = straight chain (10)
sec = 20 , tert = 30
iso = 10 ( if second last carbon contains one methyl group and no other branches)
neo = 10 ( if second last carbon contains two methyl groups and no other branches)
Polyhalogen compounds
Polyhalogen Compounds are organic compounds containing more than one halogen atom in their molecules known as polyhalogen compounds. For an example:

Note: Vicinal dihalide : compounds that have halogens on adjacent carbon atoms.
Isomerism in Haloalkane
Isomerism is a phenomenon where compounds have the same molecular formula but different in the arrangement of atoms. Haloalkanes are derived from alkanes by substituting one or more hydrogen atoms with halogen atoms (F, Cl, Br, I). Haloalkanes show chain isomerism and position isomerism.
1. Chain isomerism
Haloalkanes having 4 or more carbon atoms show isomerism in which isomers differ like the carbon chain. For an example:

2. Position isomerism
Haloalkanes having 3 or more carbon atoms show position isomerism in which isomers differ in the position of halogen atoms. For Example:

Preparation of haloalkanes
A. Preparation of Haloalkanes from Alcohol
1. By the action of halogen acids:
Alcohols can be converted into haloalkanes by treating them with halogen acids in the presence of a dehydrating agent such as anhydrous zinc chloride or conc. H2SO4.

Where, HX = HCl, HBr, HI
The reactivity of halogen acids follows the order :
HCl < HBr < HI
It is because of the fact that bond dissociation energy is : HCl>HBr>HI.
Reactivity of alcohols towards this reaction is :
Primary<Secondary<Tertiary.
This is because the greater the number of electron-releasing groups on α- carbon atom of alcohol, the greater the polarity of the C – OH bond. The greater the polarity of the C –OH bond, the more reactive the alcohol.
Examples :

Note : The mixture of HCl and ZnCl2 is known as “Lucas reagent”.

2. By the action of phosphorus halides:
Alcohols on refluxing with phosphorus penta or trihalides give haloalkane.

For example :

Note :
H3PO3 = phosphorous acid
H3PO4 = phosphoric acid
POCl3 = phosphorus oxychloride ( IUPAC = Phosphoryl trichloride )
3. By the action of thionyl chloride:
Alcohols when refluxed with thionyl chloride in the presence of pyridine give chloroalkane.

Note : SOBr2 and SOI2 are unstable, therefore bromo and iodo alkanes can not be prepared by this method.
B. Preparation of haloalkanes from hydrocarbons
1. From alkanes:
- Alkanes when treated with a limited amount of halogen in the presence of heat, light, or a suitable catalyst give haloalkanes.

- If the excess of halogen is used then a mixture of mono and polysubstituted products is obtained.

- Bromination also similarly takes place.
- Iodination is reversible thus the reaction is always carried out in the presence of an oxidizing agent like HIO3, conc. HNO3, etc. The use of an oxidizing agent is to oxidize ‘HI’ formed during the reaction into ‘I2’ and hence shifts the equilibrium in a forward direction.

2. From alkenes: By the action of halogen acids (HCl, HBr, and HI)
Alkene reacts with halogen acids to give alkyl halide (haloalkane). Eg.

When alkene is unsymmetrical then the addition takes place according to Markovnikov’s rule.
Physical properties of haloalkanes
1. Physical state: The lower members of alkyl halide are gaseous at room temperature (up to C5) and higher members of alkyl halide are colorless liquid or solid.
2. Boiling point: The boiling points of haloalkanes having the same alkyl group follow the order :
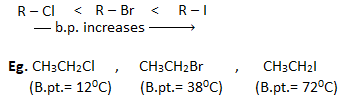
This is because, with the increase in size and mass of the halogen atom, the magnitude of Vander Waal’s force increases, and hence boiling point also increases.
- For the alkyl halides having the same halogen atoms boiling point increases with the increase in size of an alkyl group. Eg.

- With the increase in the branching of an alkyl group, the surface area decreases and the magnitude of Vander Waal’s force also decreases. Hence, the boiling point of isomeric alkyl halides decreases with the increase in the branching of the alkyl group.
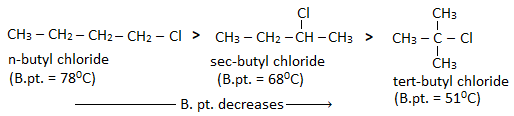
Haloalkanes have higher boiling points than alkanes of comparable molecular masses. This is because haloalkanes are polar and due to their polarity, a strong dipole-dipole interaction exists between the molecules of haloalkanes.

3. Solubility :
Although haloalkanes are polar in nature, they are insoluble in water because they are not able to form hydrogen bonds with water molecules. However, they are soluble in organic solvents like benzene, ether, alcohol, etc.
Chemical properties of haloalkanes
- Nucleophilic substitution reaction.
- Elimination reaction
- Reaction with metals
- Reduction reaction
1. Nucleophilic substitution reaction in haloalkanes
A nucleophilic substitution reaction is a chemical reaction that involves the displacement of a leaving group by a nucleophile. In this process, the leaving group i.e. the halogen atom departs along with the bonding pair of electrons, and the electrons for the formation of the new bond are furnished (provided/supplied) by the nucleophile.

It is of two types;
i. SN1 reaction:
- SN1 indicates the unimolecular nucleophilic substitution reaction.
- The rate of SN1 reaction depends only upon the concentration of the substrate.
i.e. Rate = k[Substrate]
- The reaction occurs in two steps. In the first step, carbocation is formed and in the second step, nucleophiles attack the carbocation to give a substituted product.
Example:

- The rate of the reaction is directly proportional to the stability of carbocations. Hence, the order of reactivity is 30 > 20 > 10 haloalkanes.
ii. SN2 reaction:
- SN2 indicates the bimolecular nucleophilic substitution reaction.
- The rate of SN2 reaction depends upon the concentration of both substrate and nucleophile.
i.e. Rate = k[Substrate][:Nu –]
- The reaction occurs in a single step, SN2 reaction occurs through a transition state as shown below: Example:

- The rate of reaction is inversely proportional to the bulkiness of groups attached to the C atom. Hence, the order of reactivity is 10 > 20 > 3O haloalkanes.
1. Substitution by hydroxyl group ( formation of alcohols):
When a haloalkane is boiled with an aqueous solution of KOH or moist silver oxide (Ag2O), gives alcohol.
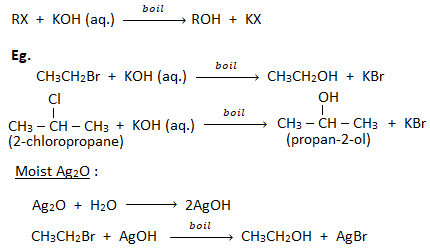
2. Substitution by cyano group ( formation of cyanides or nitriles):
When haloalkane is treated with alcoholic KCN solution it gives alkane nitrile ( or alkyl cyanide) as the major product.

The alkyl cyanide so produced can be used as the starting material for the preparation of a number of other compounds.
- Partial hydrolysis with conc. HCl or alkaline solution of H2O2 gives amide.

- Complete hydrolysis with dil. HCl gives carboxylic acid.

- Reduction with nascent hydrogen produced by LiAlH4 (Lithium aluminum hydride), Na/alcohol, or H2/Ni gives primary amine.


3. Substitution by isocyanide (i.e. – NC ) (formation of isocyanide):
Alkyl halide when treated with an alcoholic solution of silver cyanide (AgCN) gives alkyl isocyanide.

Alkyl isocyanide on reduction gives secondary amine.


4. Substitution by alkoxy group (Formation of ether):
Alkyl halide when treated with sodium or potassium alkoxide gives ether. This reaction is known as Williamson’s ether synthesis. Example:

5. Substitution by amino group (Formation of amines):
When a mixture of haloalkane and alc. NH3 is heated which gives primary amine.

If the haloalkane is present in excess, secondary and tertiary amines are formed. Finally, tertiary amine reacts with haloalkane to give quaternary ammonium salt as the final product.

6. Substitution by nitro group (-NO2) : [Formation of nitroalkane]
Alkyl halide when treated with an alcoholic solution of silver nitrate (AgNO2) gives nitroalkane.

7. Substitution by nitrite group (-O-N=O) : [Formation of alkyl nitrite]
Alkyl halide reacts with sodium or potassium nitrite to form alkyl nitrite.

Note :
2. Elimination reaction
- When an alkyl halide is heated with an alcoholic solution of KOH, then a molecule of hydrogen halide is eliminated from the haloalkane, and alkene is formed. Therefore this reaction is also called dehydrohalogenation reaction. Example:
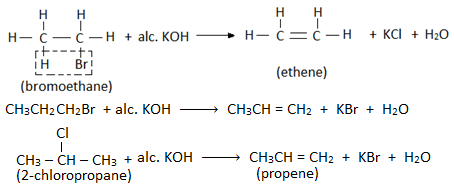
- Elimination reaction involves the removal of a halogen atom of haloalkane and a hydrogen atom from the β- carbon (i.e. adjacent carbon). Therefore, this reaction is also known as β- elimination reaction.
- If two or more than two elimination products can be obtained from an alkyl halide then elimination takes place according to Saytzeff’s rule.
Saytzeff’s rule ( or Zaitsev’s rule)
When two β- carbon atoms are present, then the elimination of the H-atom takes place from the β-carbon atom with less number of H- atoms i.e. highly substituted alkene is the major product of dehydrohalogenation reaction. Example:

3. Reaction of haloalkanes with metals
1. Reaction of haloalkanes with Na (Wurtz reaction):
When an alkyl halide( haloalkane) is heated with sodium metal in the presence of dry ether, a symmetrical alkane containing double the number of carbon atoms than haloalkane is formed. This reaction is called the Wurtz reaction.

This is not a good method to prepare unsymmetrical alkane because a mixture of two different haloalkanes has to be used which gives a mixture of three different alkanes.
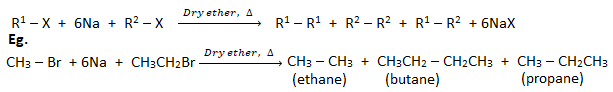
2. Reaction with magnesium:
Alkyl halide when treated with magnesium in the presence of dry ether gives alkyl magnesium halide which is known as Grignard reagent.

4. Reduction reaction of haloalkanes
Haloalkane on reduction gives the corresponding alkane. Reduction of haloalkane can be carried out by using reducing agents like:
(a) Zn/HCl,
(b) Sn/HCl,
(c) Na/ethanol,
(d) H2/Ni,
(e) Lithium aluminum hydride (LiAlH4),
(f) Zn-Cu couple,
(g) HI/Red phosphorus.
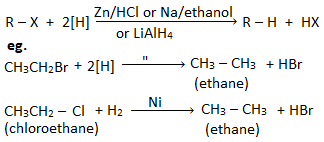
Thanks for being part of Scholarsyatra!