Introduction to Aromatic Hydrocarbons
Aromatic hydrocarbons are a class of organic compounds characterized by the presence of one or more benzene rings. The aromatic hydrocarbons are “unsaturated hydrocarbons which have one or more planar six-carbon rings called benzene rings, to which hydrogen atoms are attached”. These compounds play a crucial role in both synthetic and natural chemistry and are fundamental building blocks in pharmaceuticals, polymers, dyes, and other materials. Aromatic hydrocarbons differ significantly from aliphatic hydrocarbons (alkanes, alkenes, and alkynes) due to their unique structure and chemical behavior.
Table of Contents
Toggle
Definition
Aromatic hydrocarbons (also called arenes) are hydrocarbons that contain at least one aromatic ring. The simplest and most important member of this class is benzene (C₆H₆), which serves as the prototype for all aromatic compounds.
History and Discovery of Aromatic Hydrocarbons
The term “aromatic” was initially used to describe a range of fragrant compounds. However, with time, it was observed that many of these “aromatic” compounds contained a common structural feature – the benzene ring – regardless of whether they were fragrant. Thus, the term “aromatic” took on a structural, rather than a fragrance-related meaning in chemistry.
Benzene was first discovered by Michael Faraday in 1825, and its structure was proposed by Friedrich August Kekulé in 1865 as a ring of six carbon atoms connected by alternating single and double bonds.
Structure of Aromatic Hydrocarbons
The core structural feature of aromatic hydrocarbons is the aromatic ring, typically a benzene ring in its simplest form. Aromatic hydrocarbons may contain:
- Monocyclic Aromatic Hydrocarbons: A single aromatic ring, such as in benzene (C₆H₆).
- Polycyclic Aromatic Hydrocarbons (PAHs): Multiple fused aromatic rings, like in naphthalene (C₁₀H₈) or anthracene (C₁₄H₁₀).
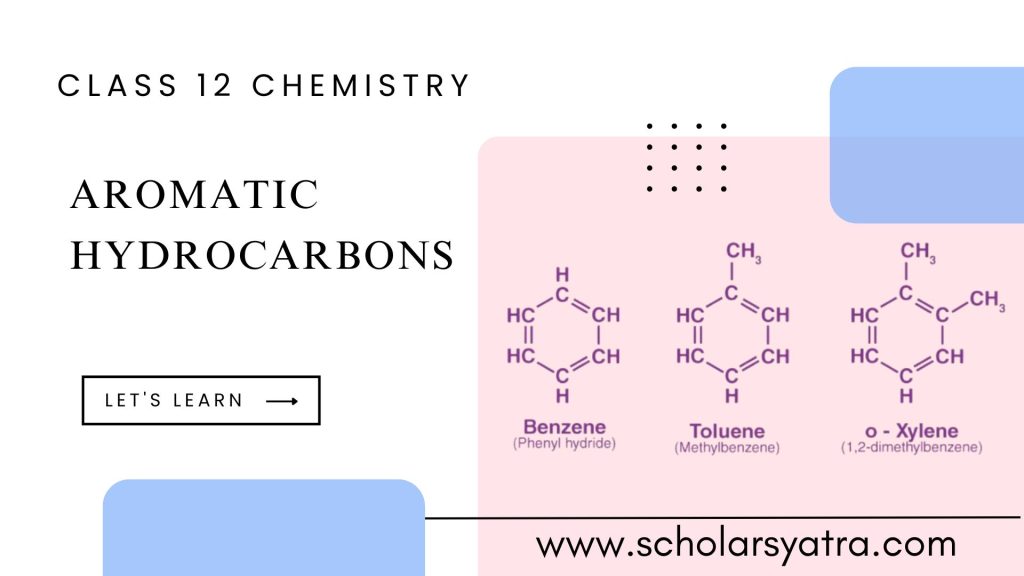
Structure of Benzene (C₆H₆)
Benzene consists of six carbon atoms arranged in a planar hexagonal ring, with each carbon atom bonded to a single hydrogen atom. The unique feature of benzene is the delocalization of π-electrons over the entire ring, which gives benzene its stability. This electron delocalization is a result of resonance, where the positions of double bonds alternate between different carbon-carbon pairs without altering the overall molecular structure.
In benzene:
- The C-C bond lengths are equal (~1.39 Å), intermediate between a typical single bond and a double bond.
- The molecule is planar and exhibits sp² hybridization at each carbon atom, creating a continuous overlap of p-orbitals, which results in a delocalized π-electron cloud above and below the plane of the ring.
Huckel’s Rule
A compound is considered aromatic if it satisfies Hückel’s Rule, which states that a molecule must:
- Be cyclic.
- Be planar.
- Have a fully conjugated system of p-orbitals (every atom in the ring must have a p-orbital).
- Contain a number of π-electrons equal to 4n+24n+24n+2, where nnn is a non-negative integer. For benzene, the number of π-electrons is 6 (as n=1n = 1n=1).
Nomenclature of Aromatic Hydrocarbons
Aromatic hydrocarbons can have various substituents attached to the ring, and their nomenclature is based on IUPAC rules. Some common rules include:
- Simple Aromatic Compounds: The simplest aromatic hydrocarbon is benzene. If one substituent is attached, the name of the compound is based on the substituent followed by “benzene” (e.g., chlorobenzene, nitrobenzene).
- Disubstituted Benzene: For two substituents on the benzene ring, their relative positions are denoted using prefixes:
- Ortho (o-): Substituents at adjacent carbons (1,2 positions).
- Meta (m-): Substituents separated by one carbon (1,3 positions).
- Para (p-): Substituents opposite each other on the ring (1,4 positions).
- Polysubstituted Benzene: If more than two substituents are present, the positions of the substituents are indicated by numbers, and the names are arranged in alphabetical order.
Types of Aromatic Hydrocarbons
1. Monocyclic Aromatic Hydrocarbons
- Benzene (C₆H₆): The simplest aromatic hydrocarbon.
- Toluene (C₆H₅CH₃): Benzene with a methyl group attached.
- Phenol (C₆H₅OH): Benzene with a hydroxyl group attached.
2. Polycyclic Aromatic Hydrocarbons (PAHs)
PAHs consist of two or more fused aromatic rings. Examples include:
- Naphthalene (C₁₀H₈): Consists of two fused benzene rings.
- Anthracene (C₁₄H₁₀) and Phenanthrene (C₁₄H₁₀): Both consist of three fused benzene rings in different arrangements.
These compounds are often found in substances like coal tar, and petroleum, and can be environmental pollutants due to their persistence and potential toxicity.
Physical Properties of Aromatic Hydrocarbons
- Physical State: Most aromatic hydrocarbons are liquids or solids at room temperature. Benzene, toluene, and xylenes are common liquids, while polycyclic aromatic hydrocarbons like naphthalene are solids.
- Aromaticity and Stability: The delocalization of π-electrons provides aromatic compounds with significant stability (resonance energy). This makes them less reactive than alkenes or alkynes in addition reactions but more reactive in substitution reactions.
- Solubility: Aromatic hydrocarbons are generally non-polar, making them insoluble in water but soluble in organic solvents such as ethers, alcohols, and benzene.
- Melting and Boiling Points: Aromatic hydrocarbons have higher melting and boiling points compared to aliphatic hydrocarbons of similar molecular weight due to their planarity and strong van der Waals forces.
Chemical Properties of Aromatic Hydrocarbons
Aromatic hydrocarbons exhibit a unique set of chemical reactions due to the stability of their π-electron system. The most common reactions they undergo are electrophilic aromatic substitution (EAS) reactions, where a hydrogen atom in the aromatic ring is substituted by another group.
1. Electrophilic Aromatic Substitution (EAS)
In EAS, an electrophile reacts with the π-electron-rich aromatic ring. Key steps include:
- Formation of the electrophile: An electrophile is generated by a catalyst.
- Arenium ion intermediate: The aromatic ring temporarily loses its aromaticity as the electrophile bonds to the ring, forming a carbocation intermediate.
- Restoration of Aromaticity: Aromaticity is restored as a proton is lost from the intermediate.
Common types of EAS reactions include:
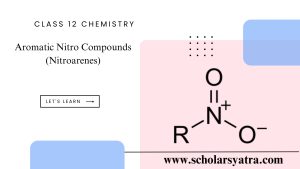
- Nitration: Introduction of a nitro group (-NO₂) using a mixture of concentrated nitric acid and sulfuric acid.
- Sulfonation: Introduction of a sulfonic acid group (-SO₃H) using sulfuric acid.
- Halogenation: Introduction of a halogen (Cl or Br) using a Lewis acid catalyst (FeCl₃ or FeBr₃).
- Friedel-Crafts Alkylation: Introduction of an alkyl group using an alkyl halide and a Lewis acid catalyst.
- Friedel-Crafts Acylation: Introduction of an acyl group (-CO-R) using an acyl halide and a Lewis acid.
2. Other Reactions
- Oxidation: Alkyl-substituted aromatic hydrocarbons can undergo oxidation, with the side chain being converted into a carboxylic acid group (-COOH).
- Hydrogenation: Under strong conditions, benzene can be hydrogenated to form cyclohexane.
Applications of Aromatic Hydrocarbons
Aromatic hydrocarbons have extensive industrial and commercial applications:
- Pharmaceuticals: Many drugs are based on aromatic structures, including aspirin (acetylsalicylic acid) and paracetamol.
- Polymers: Benzene and its derivatives are used in the production of polymers like polystyrene.
- Dyes and Pigments: Aromatic compounds, especially polycyclic aromatic hydrocarbons, form the basis of many synthetic dyes.
- Solvents: Benzene, toluene, and xylene are commonly used as industrial solvents.
- Fuel Additives: Toluene and xylene are used in high-octane fuels.
Environmental and Health Concerns
Many aromatic hydrocarbons, particularly polycyclic aromatic hydrocarbons (PAHs), are environmental pollutants. PAHs are produced during incomplete combustion of organic matter (e.g., coal, wood, petroleum) and are known to be toxic and carcinogenic. Benzene, in particular, is classified as a human carcinogen and exposure to high levels can cause leukemia.
Aromatic hydrocarbons are a fundamental class of organic compounds, distinguished by their stability due to resonance and delocalized π-electrons. Their chemical behavior, particularly in electrophilic substitution reactions, is crucial in many industrial processes, including the manufacture of drugs, dyes, and polymers. However, environmental and health concerns associated with some aromatic hydrocarbons necessitate careful handling and control, especially in industrial applications.