Introduction to Zinc
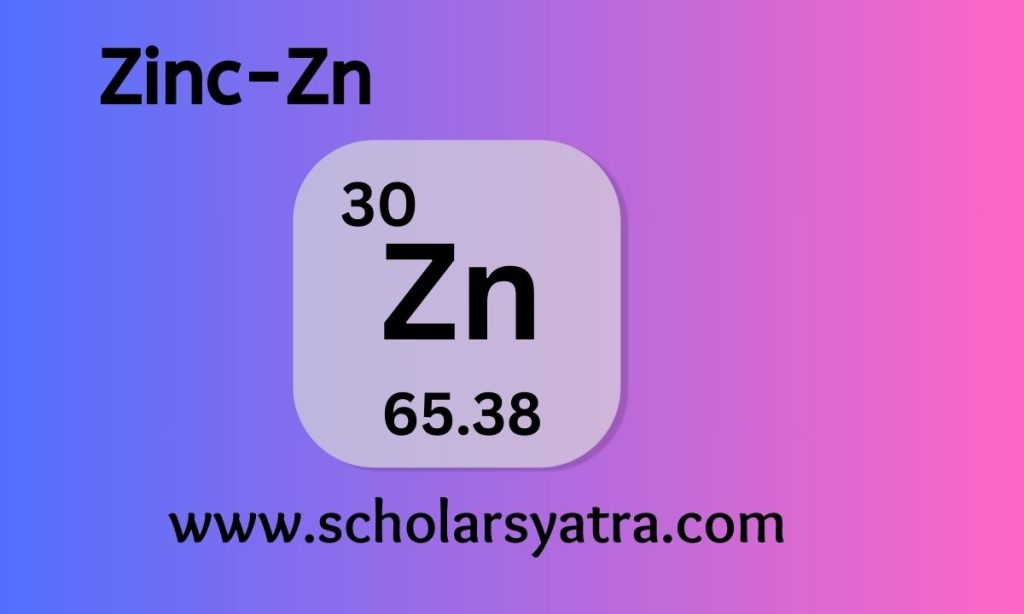
Zinc (Zn), with atomic number 30, is a transition element/metal recognized for its vital role in both industrial applications and biological systems. Zinc is one of the most abundant and widely used metals, indispensable in galvanization, alloy production, and enzyme function. As a chemistry scholar, a detailed understanding of zinc’s properties, reactions, and applications will provide deep insight into its significant contributions to both modern industry and biological processes.
Table of Contents
TogglePosition in the Periodic Table
- Atomic number: 30
- Symbol: Zn
- Block: d-block (transition metals)
- Period: 4
- Group: 12 (with cadmium and mercury)
Zinc, a Group 12 metal, sits alongside cadmium and mercury in the periodic table. With an electron configuration of [Ar] 3d¹⁰ 4s², zinc behaves as a relatively stable metal, primarily exhibiting a +2 oxidation state, which is typical in its compounds. Zinc’s chemical behavior lacks the variable oxidation states commonly seen in transition metals but retains enough reactivity to participate in various chemical reactions.
Physical Properties of Zinc
Zinc is a bluish-white, shiny metal with distinctive physical properties:
- Atomic mass: 65.38 u
- Melting point: 419.53°C (787.15°F)
- Boiling point: 907°C (1665°F)
- Density: 7.14 g/cm³
- Electrical conductivity: Moderate conductor of electricity
- Crystal structure: Hexagonal close-packed (hcp)
At room temperature, zinc is brittle, but it becomes malleable between 100°C and 150°C, making it easier to shape and work with. Zinc’s low melting point makes it ideal for casting in various industrial applications. Additionally, zinc forms a protective oxide layer when exposed to air, giving it excellent corrosion resistance.
Chemical Properties of Zinc
Zinc is moderately reactive and has a variety of important chemical characteristics. It exhibits a stable +2 oxidation state, forming ionic bonds in most of its compounds.
- Oxidation State: Zinc typically forms the Zn²⁺ ion, a stable oxidation state found in nearly all of its compounds.
- Reaction with Acids: Zinc readily reacts with dilute acids, such as hydrochloric acid, to produce hydrogen gas and a corresponding zinc salt:
Zn+2HCl→ZnCl2+H2
This reaction illustrates the metal’s reducing power and is commonly used in laboratories to generate hydrogen gas.
- Reaction with Oxygen: When heated, zinc reacts with oxygen to form zinc oxide (ZnO), a compound that finds use in many industries:
2Zn+O2→2ZnO
Zinc oxide is amphoteric, meaning it can react with both acids and bases, forming zinc salts or complex ions such as zincates.
- Amphoteric Nature: Zinc oxide behaves as both an acid and a base:
- With hydrochloric acid, it forms zinc chloride:ZnO+2HCl→ZnCl2+H2O
- With sodium hydroxide, it forms sodium zincate:ZnO+2NaOH+H2O→Na2[Zn(OH)4]
- Reactivity with Halogens: Zinc reacts with halogens to form zinc halides (e.g., zinc chloride, ZnCl₂), which are used in various chemical processes.
- Complex Ion Formation: Zinc forms stable complexes with ligands such as ammonia and cyanide, giving rise to coordination complexes like [Zn(NH₃)₄]²⁺.
The compounds of zinc are:
Hydrides
The term hydride is used to indicate compounds of the type MxHy and it is not necessarily that indicated any compounds listed would behave as same as hydrides chemically.
- Zinc hydride: ZnH2
Fluorides
- Zinc difluoride: ZnF2
Chlorides
- Zinc dichloride: ZnCl2
Bromides
- Zinc dibromides: ZnBr2
Iodides
- Zinc diiodide: ZnI2
Oxides
- Zinc oxide: ZnO
- Zinc peroxide: ZnO2
Sulfides
- Zinc sulphide: ZnS
Selenides
- Zinc selenide: ZnSe
Tellurides
- Zinc telluride: ZnTe
Nitrides
- Trizinc dinitride: Zn3N2
Complexes
- Hexaaquozinc dinitrate: Zn(NO3)2.6H2O
- Zinc sulphate pentahydrate: ZnSO4.7H2O
Ores of Zinc
Calamine → ZnCO3
Zincite → ZnO
Zinc blende → ZnS
Willemite → ZnO.SiO2
Extraction and Occurrence
Zinc is the 24th most abundant element in the Earth’s crust. It is primarily found in the form of zinc blende (sphalerite, ZnS), and other important minerals like smithsonite (ZnCO₃) and hemimorphite (Zn₄Si₂O₇(OH)₂·H₂O).
Zinc Extraction Process:
- Mining: Zinc is obtained from its ore, sphalerite, through underground or open-pit mining.
- Roasting: Zinc sulphide is roasted to produce zinc oxide and sulphur dioxide:2ZnS+3O2→2ZnO+2SO2
- Reduction (Pyrometallurgical Method): Zinc oxide is reduced with carbon at high temperatures to produce zinc metal:ZnO+C→Zn+CO
- Electrolytic Refining: In hydrometallurgical processes, zinc is dissolved in sulfuric acid and recovered by electrolysis.
Uses of Zinc
Zinc has a broad range of applications across many industries due to its unique properties:
1. Galvanization
Zinc is most commonly used for galvanizing iron and steel to protect them from rusting. Galvanized steel is used in infrastructure, automotive industries, and household appliances. The zinc coating acts as a protective layer and corrodes preferentially over iron, thereby preventing oxidation of the underlying metal.
2. Alloys
Zinc is a key component in a variety of alloys, such as:
- Brass: An alloy of copper and zinc, used for its durability, corrosion resistance, and aesthetic appeal. Brass is used in musical instruments, plumbing fixtures, and decorative objects.
- Zamak: A zinc-aluminum alloy used in die casting, commonly found in automotive parts, hardware, and electrical components.
3. Zinc Oxide (ZnO)
Zinc oxide is used in:
- Rubber Industry: As an additive to vulcanize rubber and improve its strength.
- Cosmetics and Skincare: ZnO is a key ingredient in sunscreens, ointments, and cosmetics due to its UV-absorbing properties.
- Pigments: Used in paints, coatings, and ceramics for its white pigmentation and anti-corrosive properties.
- Electronics: ZnO is a semiconductor used in varistors and as a transparent conductor in touch screens.
4. Batteries
Zinc is used in various types of batteries:
- Zinc-carbon batteries: Common in household batteries.
- Zinc-air batteries: Widely used in hearing aids and other portable devices.
5. Biological Significance
Zinc is essential in biology. It is a trace element necessary for the proper function of over 300 enzymes and is crucial for:
- Enzyme Activity: It acts as a cofactor for enzymes involved in DNA synthesis, cell division, and protein synthesis.
- Immune Function: Zinc plays a vital role in maintaining immune system function.
- Growth and Development: Zinc is essential for proper growth, especially during childhood and adolescence.
Zinc deficiency can lead to impaired immune function, growth retardation, and delayed wound healing. Foods rich in zinc include meat, dairy, seeds, and legumes.